
Eusebio Juaristi, Curriculum Vitae
Nació en Querétaro, México, el 21 de diciembre de 1950 y estudió química en el Instituto Tecnológico y de Estudios Superiores de Monterrey obteniendo el grado de Licenciado en Ciencias Químicas en 1972. Posteriormente obtuvo un Doctorado en Química (Ph. D., 1977) en la Universidad de Carolina del Norte en Chapel Hill, NC, EUA, bajo la supervisión de Ernest L. Eliel.
A continuación, Juaristi fue investigador asociado posdoctoral en la Universidad de California en Berkeley (1977-1978, en el grupo de Andrew Streitwieser), así como en la División de Diagnósticos de Syntex, Palo Alto, California (1978-1979), antes de regresar en 1979 a México, donde actualmente es Profesor Titular Emérito de Química en el Departamento de Química del Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV-IPN). Juaristi fue profesor visitante en el Instituto Politécnico Federal Suizo en Zurich (ETH-Zurich), Suiza, durante 1985-1986 y durante 1992-1993, en la Universidad de California en Berkeley, donde fue profesor de un curso de fisicoquímica orgánica para estudiantes de pregrado destacados (1999-2000) y en la Universidad Técnica (RWTH) de Aachen, Alemania (mayo-julio de 2013).
Contribuciones científicas
Como iniciador en México del área de la fisicoquímica orgánica con énfasis en el análisis conformacional y estereoquímica, Juaristi ayudó a desarrollar un tema de investigación previamente ausente en México.
Esta área de investigación es muy importante para comprender los mecanismos fundamentales responsables de la reactividad presentada por moléculas orgánicas y los sistemas biológicos. Por ejemplo, el efecto anomérico es el fenómeno estereoelectrónico responsable de la ruta seguida en una gran variedad de reacciones químicas, así como de la conformación adoptada por los azúcares y otras moléculas de interés biológico.
Efectivamente, Juaristi se convirtió en un líder mundial en el estudio del efecto anomérico, y ha escrito cuatro revisiones fundamentales en el campo: (1) "Recent Studies of the Anomeric Effect", Tetrahedron, Report No. 315, Pergamon Press: Oxford (1992), (2) "The Anomeric Effect", CRC Press: Boca Raton, Fl, 1995. (3) "Manifestation of Stereoelectronic Interactions in One-Bond 1JC-H Coupling Constants", Acc. Chem. Res., 40, 961-970 (2007), y (4) "Anomeric Effect in Saturated Heterocyclic Rings", en "Advances in Heterocyclic Chemistry", (A. R. Katritzky, Editor), Elsevier: Amsterdam, vol. 105, p. 189-222 (2012).

Juaristi también ha destacado en el ámbito de la síntesis asimétrica, que es de vital importancia para la industria química, en particular las industrias farmacéuticas, agroquímicas y de alimentos, ya que la síntesis asimétrica es esencial para la preparación de medicamentos biológicamente activos, alimentos y herbicidas. De hecho, el desarrollo de nuevos métodos para la síntesis enantioselectiva de b-aminoácidos por el grupo de investigación dirigido por Juaristi ha dado lugar a varias invitaciones para escribir artículos de revisión y libros en el campo: (1) "Enantioselective Synthesis of ß-Amino Acids", Aldrichim. Acta, 27, 3-11 (1994), (2) "Enantioselective Synthesis of ß-Amino Acids", Wiley-VCH: Nueva York, 1997, (3) "Recent Advances in the Enantioselective Synthesis of ß-Amino Acids", Curr. Topics Med. Chem., 6, 983-1004 (1999), (4) "Second Edition of Enantioselective Synthesis of ß-Amino Acids", Wiley: Nueva York, 2005, y (5) "Recent Advances in the Synthesis of ß-Amino Acids", en "Amino Acids, Peptides and Proteins", A. B. Hughes, Ed, Wiley-VCH: Weinheim (2009), pp 291-366.
 |
 |
Otras áreas de la química, donde Juaristi ha tenido una influencia significativa son
(1) Estructura y reactividad de los carbaniones [ver su aportación en "Comprehensive Carbanion Chemistry", Elsevier: Amsterdam, 1980, el "Feature Article" en Synthesis, 1271-1290 (1993), la comunicación en Org. Lett., 2, 3739-3742 (2000), y el capítulo titulado a-Sodium Carboxylic Acids and Other Na-C-CXYZ Compounds”, que fue publicado en la prestigiosa enciclopedia Science of Synthesis, Thieme Verlag: Stuttgart (2006).
(2) Análisis conformacional [ver su artículo de revision en Acc. Chem. Res., 22, 357 (1989), así como el libro editado por él “Conformational Behavior of Six-Membered Rings”, VCH: New York, 1995, y el artículo "Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular a-Effect", J. Am. Chem. Soc., 139, 10799-10813 (2017)],
(3) Aplicaciones de la Química computacional (ver su aportación en Encyclopedia of Computational Chemistry, Wiley: New York, 1998 y el artículo de revisión “Calorimetric and Computational Study of Sulfur-Containing Six-Membered Rings”, Chem. Soc. Rev., 34 347 (2005), y los artículos (a) "Theoretical Evidence for the Relevance of n(F) → s*(C–X) (X = H,C,O,S) Stereoelectronic Interactions", J. Org. Chem., 81, 1192-1197 (2016) y (b) "Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular a-Effect", J. Am. Chem. Soc., 139, 10799-10813 (2017).
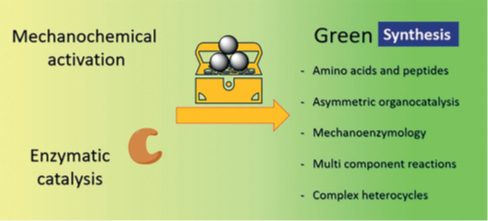
(4) Organocatálisis asimétrica y Química “verde” (ver el “feature article” “Recent Efforts Directed to the Development of More Sustainable Asymmetric Organocatalysis”, Chem. Commun., 48, 5396 (2012). El capítulo “Useful Chemical Activation Alternatives in Solvent-Free Organic Reactions”, en “Comprehensive Organic Synthesis-II”, G. A. Molander y P. Knochel, Eds., Vol. 9, Oxford: Elsevier (2014); pp. 287-314. El capítulo “Asymmetric Organocatalytic Reactions Under Ball Milling”, en “Ball Milling Towards Green Synthesis: Applications, Projects, Challenges”, R. Brindaban y A. Stolle, Editors, Royal Society of Chemistry: Cambridge, UK, (2015); capítulo 4, pp. 81-95. El artículo de revisión "The Diamino Analogs of Priviledged Corey-Bakshi-Shibata and Jorgensen-Hayashi Catalysts. A Comparison of Their Performance", Synthesis, 48, 3890-3906 (2016). Así como el artículo "Improving the Catalytic Performance of (S)-Proline as Organocatalyst in Asymmetric Aldol Reactions in the Presence of Solvate Ionic Liquids. Involvement of a Supramolecular Aggregate", Org. Lett., 19, 1108-1111 (2017). Y los artículos de revision "Recent Applications of Mechanochemistry in Enantioselective Synthesis", Tetrahedron Lett. Digest, 60, 1749-1757 (2019), "Mechanochemical and Mechanoenzymatic Synthesis of Pharmacologically Active Compounds: A Green Perspective", ACS Sustainable Chemistry & Engineering, 8, 8881-8893 (2020), "Novel methodologies for chemical activation in organic synthesis under solvent-free reaction conditions", Molecules, 25, 3579 (2020), “Mechanoenzymology: State of the Art and Challenges towards Highly Sustainable Biocatalysis”, ChemSusChem, 14, 2682-2688 (2021), “Recent Developments in Next Generation (S)-Proline-Derived Chiral Organocatalysts”, Tetrahedron Report, (2021) y “Salient Achievements in Synthetic Organic Chemistry Enabled by Mechanochemical Activation”, Synthesis, 55, 2439-2459 (2023).
 |
 |
Al día de hoy, Juaristi es autor o coautor de 471 publicaciones en el área de la química, incluidos 253 artículos basados en investigaciones originales, 49 capítulos de libros, 33 libros, 31 artículos de revisión, y docenas de artículos educativos. El impacto que estas publicaciones han tenido se refleja en el número de citas de otros autores, que supera 12,100 -aproximadamente 1,200 de ellas en libros. De hecho, Juaristi es uno de los químicos latinoamericanos más citados de todos los tiempos.
Educación y formación de recursos humanos
Varios libros de texto de química escritos o editados por Juaristi han tenido un impacto significativo en los niveles nacional e internacional. Por ejemplo, el libro “Introduction to Stereochemistry and Conformational Analysis” publicado por la prestigiosa Casa Editorial Wiley en 1991, sigue editándose y vendiéndose después de 30 años de su aparición.
Además, Juaristi ha contribuido a la educación de un número significativo de estudiantes de doctorado (42), maestría (24) y de licenciatura (61). Cabe mencionar que muchos de estos graduados son ahora investigadores independientes en diversas instituciones académicas, tanto en la Ciudad de México como en el interior de la República.

Por último, Juaristi ha impartido un extraordinario número de cursos y conferencias, prácticamente en todos los Estados de México, así como en varios países de Norte y Sur América, en Europa y en Asia. Específicamente en Argentina, Austria, Brasil, Canadá, China, Colombia, Costa Rica, Cuba, España, Finlandia, Francia, Alemania, Hungría, India, Israel, Italia, Japón, Polonia, Portugal, Puerto Rico, Rusia, Suecia, Suiza, Estados Unidos y Venezuela.
Servicios a la comunidad científica
Juaristi ha proporcionado varios servicios a la comunidad química científica, entre los que se puede mencionar su labor en la Academia Mexicana de Ciencias [(Presidente de la sección de Química, 1994-1997 y Secretario del Consejo Directivo, 2000-2002), Director de la Red Latinoamericana de Química (http://www.relaq.mx) desde su creación en 1995. Director de los Programas para “Profesores Visitantes Distinguidos” y “Estancias de Jóvenes Investigadores en Estados Unidos”, Academia Mexicana de Ciencias-FUMEC, desde el año 2002 y hasta el año 2018]. Vicepresidente (2007-2009) y presidente (2009-2011) de la Sociedad Química de México.
Miembro del consejo editorial de prestigiosas revistas científicas nacionales e internacionales [Revista Latinoamericana de Química (1983-1992), Revista de la Sociedad Química de México/Journal of the Mexican Chemical Society (1986-2011), Heteroatom Chemistry (1989-1995), Avance y Perspectiva (1996-1998), Enantiomer (1999-2002), Anales de Química, Int. Ed. (1995-1998), Current Topics in Medicinal Chemistry (2000-2020), Advances in Physical Organic Chemistry (2002-2015), Journal of Physical Organic Chemistry (2007-2020), Journal of the Brazilian Chemical Society (desde 2007), y Accounts of Chemical Research (2009-2017)].
Organizador de más de un centenar de simposios, congresos y reuniones científicas; por ejemplo, Presidente del Comité Científico de la Fifth North American Chemistry Meeting (ACS-SQM-CCS, Cancún, 1997), Presidente de la 16a International Conference on Organic Synthesis, (IUPAC, Mérida, 2006), y Presidente de la 11th Latin American Conference on Physical Organic Chemistry (IUPAC, Riviera Maya, 2011).
Premios y distinciones
Recibió el Premio de la Academia de la Investigación Científica (ahora Academia Mexicana de Ciencias) para jóvenes científicos en 1988; el Premio Manuel Noriega, otorgado por la Organización de Estados Americanos (OEA, 1990); el Premio Nacional de Química "Andrés Manuel del Río" otorgado por la Sociedad Química de México, en 1994; el Premio Nacional de Ciencias y Artes (1998). En febrero de 2006 se convirtió en Miembro de "El Colegio Nacional", máxima distinción académica en México. Por otra parte, el 17 de agosto de 2009 Juaristi se convirtió en Miembro Honorario de la American Chemical Society (el único químico mexicano en recibir esta distinción). Por último, el 4 de diciembre de 2009 Juaristi fue nombrado Profesor Emérito en el Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, y en 2012 se convirtió en Miembro Titular de la Academia Mexicana de Ciencias. El mismo año recibió el prestigioso Premio Georg Forster de la Fundación Alexander von Humboldt. En 2016 fue nombrado Miembro Honorario de la International Union of Pure and Applied Chemistry (IUPAC) e Investigador Emérito del Sistema Nacional de Investigadores (CONACYT). En 2019 le fue otorgado el Premio Heberto Castillo (Ciudad de México). Premio “Crónica” 2023 en el área de Academia.
Finalmente, fue invitado por la revista Journal of Organic Chemistry a publicar un artículo de Perspectiva, “Looking for Treasure in Stereochemistry-Land. A Path Marked by Curiosity, Obstinacy, and Serendipity”, J. Org. Chem., 77, 4861-4884 (2012).

https://doi-org.10.1021/jo300195m
Así mismo, una edición conmemorativa de la revista de química Arkivoc le fue dedicado en el año 2005 para celebrar su cumpleaños 55.
http://www.arkat-usa.org/?VIEW=BROWSE&VOLUME=2005&ISSUE=6
Análisis Conformacional
Destacan sus estudios del efecto anomérico [E. Juaristi y G. Cuevas, “The Anomeric Effect”, CRC Press: Boca Raton, Fl (1995)], de las contribuciones entálpicas y entrópicas en equilibrios conformacionales [vease, por ejemplo: E. Juaristi, V. Labastida y S. Antúnez, “Enthalpic and Entropic Contributions to the Conformational Free Energies..." Journal of Organic Chemistry, 65, 969-973 (2000)] y el análisis conformacional de anillos de seis miembros [E. Juaristi, ed., “Conformational Behavior of Six-Membred Rings”, VCH: New York (1995)].
- E. Juaristi, G.A. Rosquete-Pina, M. Vázquez-Hernández y A.J. Mota, “Salt Effects on the Conformational Behavior of 5-Substituted 1,3-Dioxanes”, Pure & Appl. Chem., 75, 589-599 (2003).
- J.S. Cruz-Sánchez y E. Juaristi, “Contrasting Conformational Behavior of 5-Methylsulfonyl-1,3-dioxane and -1,3-Dithiane in the Minimization of Steric and Electrostatic Interactions”, Tetrahedron Lett., 43, 9369-9372 (2002).
- M. Vázquez-Hernández, G. Rosquete-Pina y E. Juaristi, “Salt Effects on the Conformational Behavior of 5-Carboxy- and 5-Hydroxy-1,3-Dioxane”, J. Org. Chem., 69, 9063-9072 (2004).
- M. Sosa-Rivadeneyra, L. Quintero, C. Anaya de Parrodi, E. Juaristi y S. Bernés, “N,N’-bis[(R)-2-Hydroxy-2-phenylethyl]-N,N’bis[(S)-1-phenylethyl]pyridine-2,6-dicarboxamide. Stabilization of an Asymmetric Conformer Through the Formation of a Double Intramolecular Hydrogen Bond”, Acta Cryst. Sect. E, 61, 536-538 (2005).
- M. Hernández-Rodríguez y E. Juaristi, “Synthesis and Conformational Análisis of Chiral Ureas Incorporating N-1-Phenylethyl Groups. Manifestation of Allylic 1,3-Strain”, J. Phys. Org. Chem., 18, 792-799 (2005).
- G. Huelgas, S. Bernés, M. Sánchez, L. Quintero, E. Juaristi, C. Anaya de Parrodi y P. J. Walsh, "Síntesis and Dynamics of Atropisomeric (S)-N-(α-Phenylelthyl)benzamides", Tetrahedron, 63, 12655-12664 (2007).
- E. Juaristi, "Introducción a la Estereoquímica y al Análisis Conformacional", 1000 ejemplares, El Colegio Nacional: México, 2007. ISBN: 970-640-333-7.
- E. Juaristi y Y. Bandala, “Anomeric Effect in Saturated Heterocyclic Ring Systems”, en “Advances in Heterocyclic Chemistry”, A.R. Katritzky, Editor, Elsevier: Amsterdam (2012), Vol. 105, p. 189-222
- E. Jiménez-González, C.G. Avila-Ortiz, R. González-Olvera, J. Vargas-Caporali, G. Dewynter y E. Juaristi, “Synthesis in Solution of Novel Seven-Membered Cyclic Dipeptides Containing - and -Amino Acids”, Tetrahedron, 68, 9842-9852 (2012).
- E. Juaristi y Y. Bandala, “Anomeric Effect in Saturated Heterocyclic Ring Systems”, en “Advances in Heterocyclic Chemistry”, A.R. Katritzky, Editor, Elsevier: Amsterdam (2012), Vol. 105, p. 189-222.
- E. Juaristi, “Looking for Treasure in Stereochemistry-Land. A Path Marked by Curiosity, Obstinacy and Serendipity”, J. Org. Chem. (Perspective), 77, 4861-4884 (2012).
- E. Juaristi y R. Notario, “Computational Reexamination of the Eclipsed Conformation in cis-2-tert-Butyl-5-(tert-butylsulfonyl)-1,3dioxane”, Struct. Chem., 24, 1855-1862 (2013).
- E. Juaristi, “Obras 5. Análisis Conformacional y Modelado Molecular”, El Colegio Nacional: México (2014). ISBN: 978-607-724-083-9
- E. Juaristi y R. Notario, “Theoretical Examination of the S-C-P Anomeric Effect”, J. Org. Chem., 80, 2879-2883 (2015)
- R. De Marco, A. Tolomelli, E. Juaristi y L. Gentilucci, "Integrin Ligands with a/ß-Hybrid Peptide Structure: Design, Bioactivity and Conformational Aspects", Medicinal Research Reviews, 36, 389-424 (2016).
- E. Juaristi, G. Gomes, A. Terent'ev, R. Notario y I. Alabugin, "Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular a-Effect", J. Am. Chem. Soc., 139, 10799-10813 (2017).
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One- Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One- Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- I. J. Arroyo-Córdoba, G. Gamboa-Velázquez, C. G. Ávila-Ortiz, M. A. Leyva-Ramírez, M. T. Cortez-Picasso, M. A. García-Revilla, D. E. Ramírez-Ornelas, E. Peña-Cabrera y E. Juaristi, “Structure and Conformation of Novel BODIPY Ugi Adducts”, ChemistryOpen, 11, e202200197 (2022).
- E. Juaristi, “What Is the Anomeric Effect?”, en “Oxygen, Stereoelectronic Effects”, Alabugin I, Kuhn, L, American Chemical Society, Washington, D.C., USA, las Secciones 3.8 y 3.9, que incluyen dos videos (2023).
Diseño de Organocatalizadores Quirales
Pionero en México en la síntesis de nuevos organocatalizadores quirales, así como su aplicación en síntesis asimétrica. Algunas publicaciones representativas son:
- F. García-Flores, L.S. Flores-Michel y E. Juaristi, “Asymmetric Allylation of Benzoyl-hydrazones Promoted by Novel C2-Symmetric Bis-Sulfoxide Organocatalysts”, Tetrahedron Lett., 47, 8235-8238 (2006).
- Y. Liu, R. Melgar-Fernández y E. Juaristi, “Enantioselective Amination of -Phenyl -Cyanoacetate Catalyzed by Chiral Amines Incorporating the -Phenylethyl Auxiliary”, J. Org. Chem., 72, 1522-1525 (2007).
- R. González-Olvera, P. Demare, I. Regla y E. Juaristi, “Application of (1S,4S)-2,5-Diazabicyclo[2.2.1]heptane Derivatives in Asymmetric Organocatlysis: the Biginelli Reaction”, Arkivoc, Número Especial de Homenaje al Prof. Torbjorn Norin, vi, 61-72 (2008).
- M. Hernández-Rodríguez, C.G. Avila-Ortiz, J. Martín del Campo, D. Hernández-Romero, M.J. Rosales-Hoz y E. Juaristi, “Synthesis of Novel Chiral (Thio) Ureas and their Application as Organocatalysts in Asymmetric Synthesis”, Australian J. Chem., 61, 364-375 (2008).
- J.L. Olivares-Romero y E. Juaristi, “Synthesis of Two Novel Chiral Diamines Derived from (S)-Proline and their Evaluation as Precursors of Diazaborolidines for the Catalytic Borane-Mediated Enantioselective Reduction of Prochiral Ketones”, Tetrahedron, 64, 9992-9998 (2008).
- E. Juaristi, “Organocatalizadores Quirales ysu Aplicación en Síntesis Asimétrica”, Educación Química, 22, 12-14 (2011).
- J.G. Hernández, Víctor García-López y E.Juaristi, “Solvent-Free Asymmetric AldolReaction Organocatalyzed by (S)-Proline-containingThiodipeptides under Ball-Milling Conditions”, Tetrahedron, 68, 92-97 (2012).
- J.G. Hernández y E. Juaristi, “Recent Efforts Directed to the Development of More Sustainable Asymmetric Organocatalysis”, Chem. Commun., (Feature Article), 48, 5396-5409 (2012).
- Vega-Peñaloza, O. Sánchez-Antonio, M. Escudero-Casao, G. Tasnádi, F. Fulop y E. Juaristi, “Stereoselective Synthesis of Chiral Pyrrolidine Derivatives of (+)--Pinene Containing a -Amino Acid Moiety”, Synthesis, 45, 2458-2468 (2013).
- Vega-Peñaloza, O Sánchez-Antonio, C.G. Avila-Ortiz, M. Escudero-Casao y E. Juaristi, “An Alternative Synthesis of Chiral (S)-Proline Derivatives Containing a Thiohydantoin Moiety and Their Application in the Asymmetric Michael Addition Reaction Under Solvent-Free Conditions”, Asian J. Org. Chem., 3, 487-496 (2014).
- C.G. Avila-Ortiz, M. López-Ortiz, A. Vega-Peñaloza, I. Regla y E. Juaristi, "Use of (R)-Mandelic Acid as Chiral Co-Catalyst in the Michael Addition Reaction Organocatalyzed by (1S,4S)-2-Tosyl-2,5-diazabicyclo[2.2.1]heptane under Solvent-Free Conditions", Asymmetric Catalysis, Topical Issue on Organocatalysis, 2, 37-44 (2015).
- E. Machuca, G. Granados, B. Hinojosa y E. Juaristi, "Synthesis and Evaluation of (S)-Proline-Containing Dipeptidic Organocatalysts Bound to MBHA Resin in Asymmetric Aldol Reactions", Tetrahedron Lett., 56, 6047-6051 (2015).
- G. Reyes-Ragel, J. Vargas-Caporali y E. Juaristi, "In Search of Diamine Analogs of the ,-Diphenylprolinol Priviledged Chiral Organocatalyst. Synthesis of Diamine Derivatives of ,-Diphenyl-(S)-prolinol and Their Application as Organocatalysts in the Asymmetric Michael and Mannnich Reactions", Tetrahedron, 72, 379-391 (2016).
- G. Reyes-Rangel, J. Vargas-Caporali y E. Juaristi, "In Search of Diamine Analogs of the a,a Diphenylprolinol Privileged Chiral Organocatalyst. Synthesis of Diamine Derivatives of a,a-Diphenyl-(S)-prolinol and their Application as Organocatalysts in the Asymmetric Michael and Mannich Reactions”, Tetrahedron, 72, 379-391 (2016).
- A. Obregón-Zúñiga, M. Milán y E. Juaristi, "Improving the Catalytic Performance of (S)-Proline as Organocatalyst in Asymmetric Aldol Reactions in the Presence of Solvate Ionic Liquids. Involvement of a Supramolecular Aggregate", Org. Lett., 19, 1108-1111 (2017).
- G. Reyes-Rangel, J. Vargas-Caporali y E. Juaristi, "Asymmetric Michael Addition Reaction Organocatalyzed by Stereoisomeric Pyrrolidine Sulfinamides under Neat Conditions. A Brief Study on Self-Disproportionation of Enantiomers", Tetrahedron, 73, 4707-4718 (2017).
- A. Obregón-Zúñiga, M. Guerrero-Robles y E. Juaristi, "Chiral Imidazolium Ionic Liquids Derived from (S)-Prolinamine as Organocatalysts in the Asymmetric Michael Reaction and Michael-Aldol Cascade Reaction under Solvent-Free Conditions", Eur. J. Org. Chem., 2692-2697 (2017).
- A. Obregón-Zúñiga y E. Juaristi, “(2S,4R)-Hyp-(S)-Phe-OMe Dipeptide Supported on Imidazolium Tagged Molecules as Recoverable Organocatalysts for Asymmetric Aldol Reactions Using Water as Reaction Medium", Tetrahedron, 73, 5373-5380 (2017).
- C. G. Avila-Ortiz, L. Díaz-Corona, E. Jiménez-González y E. Juaristi, "Asymmetric Michael Addition Orgnocatalyzed by a,ß-Dipeptides Under Solvent-Free Reaction Conditions", Molecules, 22, 1328-1342 (2017).
- J. Vargas-Caporali y E. Juaristi, "The Diamino Analogs of Privileged Corey-Bakshi-Shibata and Jorgensen-Hayashi Catalysts. A Comparison of Their Performance", Synthesis, 48, 3890-3906 (2016).
- Vargas-Caporali y E. Juaristi, "Determination of Enantioselectivities by Means of Chiral Stationary Phase HPLC in Order to Identify Effective Proline-Derived Organocatalysts", J. Brazilian Chem. Soc., 29, 896-915 (2018).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatlysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- C. Cruz-Hernández, E. Martínez-Martínez, P.E. Hernández-González y E. Juaristi, "Synthesis of a New N-Chiral Diamino-phosphoryl-N'-[(2S)-2-pyrrolidinylmethyl]-thiourea as an Organocatalyst for the Stereoselective Michael Addition of Cyclohexanone to Nitrostyrenes and Chalcones-Applications in Cascade Processes for the Synthesis of Polycyclic Systems", Eur. J. Org. Chem., 2018, 6890-6900 (2018).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatlysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- C. Cruz-Hernández, E. Martínez-Martínez, P.E. Hernández-González y E. Juaristi, "Synthesis of a New N-Chiral Diamino-phosphoryl-N'-[(2S)-2- pyrrolidinylmethyl]-thiourea as an Organocatalyst for the Stereoselective Michael Addition of Cyclohexanone to Nitrostyrenes and Chalcones-Applications in Cascade Processes for the Synthesis of Polycyclic Systems", Eur. J. Org. Chem., 2018, 6890-6900 (2018).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- O. Sánchez-Antonio y E. Juaristi, "Synthesis of a New Chiral Organocatalyst Derived from (S)-Proline Containing a 1,2,4-Triazolyl Moiety and Its Application in the Asymmetric Aldol Reaction. Effect of Water", Tetrahedron Lett., 60, 151128 (2019).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- O. Sánchez-Antonio y E. Juaristi, "Synthesis of a New Chiral Organocatalyst Derived from (S)-Proline Containing a 1,2,4-Triazolyl Moiety and Its Application in the Asymmetric Aldol Reaction. Effect of Water", Tetrahedron Lett., 60, 151128 (2019).
- O. Sánchez-Antonio, K.A. Romero-Sedglach, E.C. Vázquez-Orta y E. Juaristi, "New mesoporous silica-?supported organocatalysts based on (2S)?-?(1,?2,?4-?triazol-?3-?yl)?-?proline: efficient, reusable, and heterogeneous catalysts for the asymmetric aldol reaction", Molecules, 25, 4532 (2020).
- C.G. Avila-Ortiz y E. Juaristi, "Novel methodologies for chemical activation in organic synthesis under solvent-?free reaction conditions", Molecules, 25, 3579 (2020).
- J.M. Landeros, C. Cruz-Hernández y E. Juaristi, “a-Amino Acids and a,b-Dipeptides Intercalated into Hydrotalcite: Efficient Catalysts in the Asymmetric Michael Addition Reaction of Aldehydes to N-Substituted Maleimides”, Eur. J. Org. Chem. 5117-5126 (2021).
- E. Juaristi, “Recent developments in next generation (S)-proline-derived chiral organocatalysts”, Tetrahedron, 88, 132143 (2021).
- C. G. Ávila-Ortiz, A. Vega-Peñaloza y E. Juaristi, “Organocatalytic Reactions under Non-Traditional Conditions”, en “Asymmetric Organocatalysis: New Strategies, Catalysts, and Opportunities”, L. Albrecht, A. Albrecht y L. Dell'Amico (Editors), Wiley-VCH, Weinheim, capítulo 13, pp. 433-454 (2023).
-
E. Juaristi, “The 2021 Nobel Prize in Chemistry: Relevance of Asymmetric Organocatalysis and Green Chemistry”, Technology, Science and Culture - A Global Vision, Volume IV, G. S. Solís Santos y L. R. Hernández, Editores, IntechOpen, UDLAP (2023).
Estereoquímica
Pionero en México del desarrollo de la síntesis asimétrica. Publicaciones representativas:
- E. Juaristi, ed., “Enantioselective Synthesis of b-Amino Acids”, Wiley-VCH: New York, 1997,
- E. Juaristi y H. López-Ruiz, “Recent Advances in the Enantioselective Synthesis of b-Amino Acids”, Current Medicinal Chemistry, 6, 983-1004 (1999),
- “Recent Applications of a-Phenylethylamine in the Preparation of Enantiopure Compounds”, Tetrahedron: Asymmetry, 10, 2441-2495 (1999).
- G. Cuevas y E. Juaristi, “Manifestation of Stereoelectronic Effects on the Calculated C-H Bond Lengths and Coupling Constants in Cyclohexane, Six-Membered Heterocycles, and Cyclohexanone Derivatives”, J. Am. Chem. Soc., 124, 13088-13096 (2002).
- O. Muñoz-Muñiz, M. Quintanar-Audelo y E. Juaristi, “Reexamination of CeCl3 and InCl3 as Activators in the Diastereoselective Mukaiyama Aldol Reaction in Aqueous Media”, J. Org. Chem., 68, 1622-1625 (2003).
- E. Juaristi, “Quiralidad: Izquierda y Derecha en Química”, Ciencia, 56, 43-54 (2005).
- M. Hernández-Rodríguez y E. Juaristi, "Structurally Simple Chiral Thioureas as Chiral Solvating Agents in the Enantiodiscrimination of Carboxylic Acids", Tetrahedron, 63, 7673-7678 (2007).
- G. Huelgas, S. Bernés, M. Sánchez, L. Quintero, E. Juaristi, C. Anaya de Parrodi y P. J. Walsh, "Síntesis and Dynamics of Atropisomeric (S)-N-(α-Phenylelthyl)benzamides", Tetrahedron, 63, 12655-12664 (2007).
- E. Juaristi, "Introducción a la Estereoquímica y al Análisis Conformacional", 1000 ejemplares, El Colegio Nacional: México, 2007. ISBN: 970-640-333-7.
- Y. Liu, R. Melgar-Fernández y E. Juaristi,“Enantioselective Amination of -Phenyl -Cyanoacetate Catalyzed by Chiral Amines Incorporating the -Phenylethyl Auxiliary”, J. Org. Chem., 72, 1522-1525 (2007).
- E. Forró, L. Schoenstein, L. Kiss, A. Vega-Peñaloza, E. Juaristi y F. Fulop, “Direct Enzymatic Route for the Preparation of Novel Enantiomerically Enriched Hydroxylated -Amino Ester Stereoisomers”, Molecules, 15, 3998-4010 (2010)
- J.G. Hernández y E. Juaristi, “Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Dipeptides: Improved Stereoinduction under Solvent-Free Conditions”, J. Org. Chem., 76, 1464-1467 (2011).
- E. Juaristi y H. Stefani, “Introducão a Estereoquímica e a Analíse Conformacional”, Bookman Companhia Editora: Porto Alegre, Brasil (2012). ISBN 978-85-7780-966-0.
- E. Juaristi, “Moléculas Quirales en el Espacio y Homoquirales en la Tierra”, en “La Química y el Universo”, L.F. Rodríguez, Editor, El Colegio Nacional: México (2012); pp. 103-128.
- E. Juaristi, “Looking for Treasure in Stereochemistry-Land. A Path Marked by Curiosity, Obstinacy and Serendipity”, J. Org. Chem. Perspective, 77, 4861-4884 (2012).
- A. Vega-Peñaloza, O. Sánchez-Antonio, M. Escudero-Casao, G. Tasnádi, F. Fulop y E. Juaristi, “Stereoselective Synthesis of Chiral Pyrrolidine Derivatives of (+)--Pinene Containing a -Amino Acid Moiety”, Synthesis, 45, 2458-2468 (2013).
- M. Escudero-Casao, A. Vega-Peñaloza y E. Juaristi, “Enantiopure 1,2,3-Triazo-Lyl- -amino Acids via Click Cycloaddition Reaction on Racemic Alkynyl Precursors Followed by Separation of Stereoisomers”, Curr. Topics Med. Chem., 14, 1257-1270 (2014)
- E. Juaristi y C.G. Avila-Ortiz, “Síntesis Asimétrica de - y -Aminoácidos. Acercando a la Química con la Biología y la Medicina”, en “Proteínas, en la Intersección entre las Matemáticas, la Física, la Química y la Biología”, S. Gitler, E. Juaristi, L.F. Lara y A. Ortega, coordinadores, El Colegio Nacional: México (2015); pp 13-44.
- G. Reyes-Rangel, J. Vargas-Caporali y E. Juaristi, "Asymmetric Michael Addition Reaction Organocatalyzed by Stereoisomeric Pyrrolidine Sulfinamides Under Neat Conditions. A Brief Study on Self-Disproportionation of Enantiomers", Tetrahedron, 73, 4707-4718 (2017).
- M. Pérez-Venegas, G. Reyes-Rangel, A. Neri, J. Escalante y E. Juaristi, "Mechanochemical Enzymatic Resolution of N-Benzylated-ß3-Amino Esters", Beilstein J. Org. Chem., 13, 1728-1734 (2017).
- E. Juaristi y C.G. Avila-Ortiz, "Síntesis Asimétrica de a- y ß-Aminoácidos. Acercando a la Química con la Biología y la Medicina", en "Proteínas, en la Intersección entre las Matemáticas, la Física, la Química y la Biología", S. Gitler, E. Juaristi, L. F. Lara y A. Ortega, Coordinadores, El Colegio Nacional: México (2015); pp 13-44.
- J. Vargas-Caporali y E. Juaristi, "The Development of Chiral Phase Chromatography in Connection with the Enantioselective Synthesis of ß-Amino Acids", Isr. J. Chem., 57, 896-912 (2017).
- J. Vargas-Caporali, A. van der Lee, G. Dewynter y E. Juaristi, "Synthesis of Diastereomeric Pyrrolidine Sulfamides via Anchimerically Assisted Nucleophilic Substitution and Reaction", Lett. Org. Chem., 15, 352-358 (2018).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatlysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- C. Cruz-Hernández, E. Martínez-Martínez, P.E. Hernández-González y E. Juaristi, "Synthesis of a New N-Chiral Diamino-phosphoryl-N'-[(2S)-2-pyrrolidinylmethyl]-thiourea as an Organocatalyst for the Stereoselective Michael Addition of Cyclohexanone to Nitrostyrenes and Chalcones-Applications in Cascade Processes for the Synthesis of Polycyclic Systems", Eur. J. Org. Chem., 2018, 6890-6900 (2018).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatlysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- C. Cruz-Hernández, E. Martínez-Martínez, P.E. Hernández-González y E. Juaristi, "Synthesis of a New N-Chiral Diamino-phosphoryl-N'-[(2S)-2- pyrrolidinylmethyl]-thiourea as an Organocatalyst for the Stereoselective Michael Addition of Cyclohexanone to Nitrostyrenes and Chalcones-Applications in Cascade Processes for the Synthesis of Polycyclic Systems", Eur. J. Org. Chem., 2018, 6890-6900 (2018).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- O. Sánchez-Antonio y E. Juaristi, "Synthesis of a New Chiral Organocatalyst Derived from (S)-Proline Containing a 1,2,4-Triazolyl Moiety and Its Application in the Asymmetric Aldol Reaction. Effect of Water", Tetrahedron Lett., 60, 151128 (2019).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- O. Sánchez-Antonio y E. Juaristi, "Synthesis of a New Chiral Organocatalyst Derived from (S)-Proline Containing a 1,2,4-Triazolyl Moiety and Its Application in the Asymmetric Aldol Reaction. Effect of Water", Tetrahedron Lett., 60, 151128 (2019).
- M. Pérez-Venegas, A.M. Rodríguez-Treviño y E. Juaristi, “Dual Mechanoenzymatic Kinetic Resolution of (±)?-?Ketorolac", ChemCatChem, 12, 1782-1788 (2020).
- E. Juaristi, “Algunas Consideraciones Relevantes en la Medición de la Estereoselectividad Química”, en L.F. Lara, Coordinador, Capítulo 11, Páginas 221-239, El Colegio Nacional: México (2022).
- E. Juaristi, “What Is the Anomeric Effect?”, en “Oxygen, Stereoelectronic Effects”, Alabugin I, Kuhn, L, American Chemical Society, Washington, D.C., USA, las Secciones 3.8 y 3.9, que incluyen dos videos (2023).
Fisicoquímica Orgánica
Al iniciar en México el área de la fisicoquímica orgánica con énfasis en el análisis conformacional, el Dr. Juaristi ha desarrollado una línea de investigación hasta entonces ausente en nuestro país. A nivel docente, destacan sus libros:
- E. Juaristi, “Introducción a la Estereoquímica y al Análisis Conformacional”, Minal: México, 1989 y 1998,
- E. Juaristi, “Fisicoquímica Orgánica”, Minal: México, 1989 y 1998,
- E. Juaristi, “Introduction to Stereochemistry and Conformational Analysis”, Wiley: New York, 1991 y 2000.
- O. Muñoz-Muñiz y E. Juaristi, “Computational Determination of the Enthalpic and Entropic Contributions to the Conformational Preference of Monosubstituted Cyclohexanes. Molecular Mechanics, Semiempirical, Density Functional Theory Methods, and Ab Initio Calculations”, J. Phys. Org. Chem., 15, 808-819 (2002).
- G. Cuevas y E. Juaristi, “Manifestation of Stereoelectronic Effects on the Calculated Carbon-Hydrogen Bond Lengths and 1JC-H NMR Coupling Constants in Cyclohexane, Six-Membered Heterocycles, and Cyclohexanone Derivatives”, J. Am. Chem. Soc., 124, 13088-13096 (2002).
- M.V. Roux, M. Temprado, P. Jiménez, J. Zeñón Dávalos, R. Notario, R. Guzmán-Mejía y E. Juaristi, “Calorimetric and Computational Study of Thiacyclohexane-1-oxide and 1,1-Dioxide (Thiane Sulfoxide and Thiane Sulfone). Enthalpies of Formation and the Energy of the S = O Bond”, J. Org. Chem., 68, 1762-1770 (2003).
- M. Temprado, M.V. Roux, P. Jiménez, R. Guzmán-Mejía, E. Juaristi y J.S. Chicos, “Heat Capacities of Thiane Sulfones and Thiane Sulfoxide. Refining of Cp Group Values of Organosulfur Compounds and their Oxides”, Thermochimica Acta, aceptado.
- J. Manríquez, E. Juaristi, O. Muñoz-Muñiz y L.A. Godínez, “QCM Study of the Aggregation of Starburst PAMAM Dendrimers on the Surface of Bare and Thiol-Modifies Gold Electrodes”, Langmuir, 19, 7315-7323 (2003).
- M.V. Roux, M. Temprado, P. Jiménez, R. Notario, R. Guzmán-Mejía y E.Juaristi,“Calorimetric and Computational Study of 1,3-Dithiacyclohexane 1,1-Dioxide (1,3-Dithiane Sulfone)”, J. Org. Chem., 69, 1670-1675 (2004).
- M.A. Iglesias-Arteaga, E. Juaristi y F. González, “An Electrochemical Interpretation of the Mechanism of the Chemical Decarboxylation of 6-Carboxy-perhydropyrimidin-4-ones”, Tetrahedron, 60, 3605-3610 (2004).
- M. Vázquez-Hernández, G. Rosquete-Pina y E. Juaristi, “Salt Effects on the Conformational Behavior of 5-Carboxy- and 5-Hydroxy-1,3-Dioxane”, J. Org. Chem., 69, 9063-9072 (2004).
- E. Juaristi, R. Notario y M.V. Roux, “Calorimetric and Computational Study of Sulfur-Containing Six-Membered Rings”, Chem. Soc. Rev., 34, 347-354 (2005).
- M. Temprado, M. V. Roux, P. Jiménez, R. Guzmán-Mejía y E. Juaristi, "Thermophysical Study by DSC of Some Sulfur Heterocyclic Compounds: Thiane and Thiophene Derivatives", Thermochim. Acta, 441, 20-26 (2006).
- M. V. Roux, M. Temprado, P. Jiménez, R. Notario, R. Guzmán- Mejía y E. Juaristi, "Calorimetric and Computational Study of 1,4-Dithiacyclohexane 1,1-Dioxide (1,4-Dithiane Sulfone)", J. Org. Chem., 71, 2581-2586 (2006).
- E. Bustos Bustos, M.G. García Jiménez, B.R. Díaz-Sánchez, E. Juaristi y L.A. Godínez, "Determination of Dopamine in Real Simples by Liquid Chromatography with Spectrometric Detection Ensambled to Electrochemical Detection Using Covalent Modified Glassy Carbon Electrodes with Composites of Starburst PAMAM Dendrimers and Metal Nanoparticles", Talanta, 72, 1586-1592 (2007).
- E. Navarro, F.J. González, P.D. Astudillo, M. Vázquez-Hernández, M. Hernández-Rodríguez y E. Juaristi, "The Role of Alkali and Alkaline Earth Metal Ions on the Hydrolysis of 2-Ferrocenyl-1,3-dioxane in Acetonitrile Solutions", Polish J. Chem., 81, 921-930 (2007).
- G. Huelgas, S. Bernés, M. Sánchez, L. Quintero, E. Juaristi, C. Anaya de Parrodi y P. J. Walsh, "Synthesis and Dynamics of Atropisomeric (S)-N-(α-Phenylelthyl)benzamides", Tetrahedron, 63, 12655-12664 (2007).
- E. Juaristi y G. Cuevas, "Manifestations of Stereoelectronic Interactions in 1JC-H One Bond Coupling Constants", Acc. Chem. Res., 40, 961-970 (2007).
- E. Bustos Bustos, G. Reyes-Rangel, B.R. Díaz-Sánchez, J. Manríquez Rocha, E. Juaristi, T.W. Chapman y L.A. Godínez, “Minimization of Lateral Repulsive Interactions Between -Cyclodextrine Adsorbed on the Ferrocene-PAMAM Dendrimers Modified Electrodes” , J. Braz. Chem. Soc., 19, 1010-1016 (2008).
- Y. Bandala, I.A. Rivero, T. González, J. Aviña, y E. Juaristi,“Solid Phase Synthesis of Novel / -Tetrapeptides and Electrospray Ionization Mass Spectrometric Evaluation of Their Metal Cation Complexation Behavior”, J. Phys. Org. Chem., 21, 349-358 (2008).
- E. Bustos, L.A. Godínez, G. Reyes-Rangel y E. Juaristi, “Chiral Recognition of Alanine across Modified Carbon Electrodes with 3,4-Dihydroxyphenylalanine”, Electrochim. Acta, 54, 6445-6450 (2009).
- M.V. Roux, C. Foces-Foces, R. Notario, M.A.V. Ribeiro da Silva, M.d.D.M.C. Ribeiro da Silva, A.F.L.O.M. Santos y E. Juaristi, “Thermochemical Study of L-Cysteine, L-Cystine and L-Cysteine Radicals. S-S, S-H, and C-S Bond Dissociation Enthalpies”, J. Phys. Chem. A., 114, 10530-10540 (2010).
- R. Notario, M.V. Roux, C. Foces-Foces, M.A.V. Ribeiro da Silva, M.d.D.M.C. Ribeiro da Silva, A.F.L.O.M. Santos, R. Guzmán-Mejía y E. Juaristi, “Experimental and Computational Thermochemical Study of N-Benzyl-.alanine and N-Benzyl--alanine”, J. Phys. Chem., 115, 9401-9409 (2011).
- I. Robles, M.G. García, S. Solís, G. Hernández, Y. Bandala, E. Juaristi y E. Bustos, “Electroremediation of Mercury-Polluted Soil Facilitated by Complexing Agents”, Int. J. Electrochem. Sci., 7, 2276-2287 (2012).
- E. Juaristi, “The Best of Physical Organic Chemistry in Riviera Maya, México, November 20-24, 2011”, J. Phys. Org. Chem., 25, 892-893 (2012).
- E. Juaristi, “Obras 3, Fisicoquímica Orgánica”, El Colegio Nacional: México (2013), ISBN 978-607-724-063-1.
- E. Juaristi y R. Notario, “Theoretical Examination of the S-C-P Anomeric Effect”, J. Org. Chem., 80, 2879-2883 (2015).
- E. Juaristi y R. Notario, "Theoretical Evidence for the Relevance of n(F) → s*(C–X) (X = H, C, O, S) Stereoelectronic Interactions", J. Org. Chem., 81, 1192-1197 (2016).
- E. Juaristi, G. Gomes, A. Terent'ev, R. Notario y I. Alabugin, "Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular a-Effect", J. Am. Chem. Soc., 139, 10799-10813 (2017).
- Z. Wang (Editor) y U. Wille, E. Juaristi (Editores Asociados), "Encyclopedia of Physical Organic Chemistry", Wiley: New York (2017). ISBN: 978-1-118-47045-9.
- E. Juaristi y R. Notario, "Theoretical Evidence for the Relevance of n(S)→s*(C–P), s(C–S) → s*(C–P), and n(F) → s*(C–X) (X = H, C, O, S) Stereoelectronic Interactions", en "Stereochemistry and Global Connectivity: The Legacy of Ernest Eliel", H.N. Cheng, Editor, ACS Symposium Series, Volumén 1258, American Chemical Society: Washington, D.C., 2017, Chapter 1, pp. 3-18.
- R. Notario, J.Z. Dávalos, R. Guzmán-Mejía y E. Juaristi, "Gas-Phase Acidities and Basicities of Alanines and N-Benzyl-Alanines by the Extended Kinetic Method", J. Phys. Chem. A, 122, 383-389 (2018).
- Ernest Eliel", H.N. Cheng, Editor, ACS Symposium Series, Volumén 1258, American Chemical Society: Washington, D.C., 2017, Chapter 1, pp. 3-18.
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One-Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018)
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One- Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- M. Pérez-Venegas, M.M. Téllez-Cruz, O. Solorza-Feria, A. López-Munguía, E. Castillo y E. Juaristi, "Thermal and Mechanical Stability of Immobilized Candida Antartica Lipase B: an Approximation to Mechanochemical Energetics in Enzymatic Catalysis, ChemCatChem, 12, 803-811(2020).
Modelado Molecular
Muchos de los trabajos experimentales en el grupo de investigación del Dr. Juaristi se apoyan o complementan con estudios de modelado molecular, que permiten explicar los resultados observados o ayudan a proponer mecanismos de reacción. Ejemplos recientes:
- E. Juaristi, “Stable Eclipsed Conformations”, en Encyclopedia of Computational Chemistry, Wiley: New York, 1998.
- G. Cuevas, E. Juaristi y A. Vela, “Application of the DFT Method for the Calculation of Coupling Constants”, J. Phys. Chem., 103, 932-937 (1999).
- J. Escalante, M.A. González-Tototzin, J. Aviña, O. Múñoz-Múñiz y E. Juaristi, Tetrahedron, 57, 1883-1890 (2001).
- V.M. Gutiérrez, G. Reyes, O. Muñoz y E. Juaristi, “Diastereoselective Alkylation of Dianions Derived from Chiral Analogs of b-Aminopropionic Acid”, Helv. Chim. Acta, 85, 4189-4199 (2002)
- M. Sosa-Rivadeneyra, O. Muñoz-Muñiz, C. Anaya de Parrodi, L. Quintero-Cortés y E. Juaristi, “Molecular Modelling of Salt (Lithium Chloride) Effects on the Enantiosslectivity of Diethylzinc Addition to Benzaldehyde in the Presence of Chiral b-Aminoalcohols”, J. Org. Chem., 68, 2369-2375 (2003).
- K. Martínez-Mayorga, E. Juaristi y G. Cuevas, “Manifestation of Stereoelectronic Effects on the Calculated Carbon-Hydrogen Bond Lengths and One Bond 1JC-H NMR Coupling Constants Relative Aceptor Ability of the Carbonyl (C=O), Thiocarbonyl (C=S), and Methyledene (C=CH2) Groups Towards C-H Donor Bonds”, J. Org. Chem., 69, 7266-7276 (2004).
- E. Juaristi, R. Notario y M.V. Roux, “Calorimetric and Computational Study of Sulfur-Containing Six-Membered Rings”, Chem. Soc. Rev., 34, 347-354 (2005).
- R. Notario, M.V. Roux, G. Cuevas, J. Cárdenas, V. Leyva y E. Juaristi, "Computational Study of 1,3-Dithiane 1,1-Dioxide (1,3-Dithiane Sulfone). Rigorous Description of the Inversion Process and Manifestation of Stereoelectronic Effects on 1JC-H Coupling", Chem. Phys. Chem., 110, 7703-7712 (2006).
- M.V. Roux, M. Temprado, P. Jiménez, R. Notario, R. Guzmán-Mejía y E. Juaristi, "Calorimetric and Computational Study of 1,3- and 1,4-Oxathiane Sulfones", J. Org. Chem., 72, 1143-1147 (2007).
- E. Juaristi y G. Cuevas, “Manifestations of Stereoelectronic Interactions in 1JC-H One Bond Coupling Constants”, Acc. Chem. Res., 40, 961-970 (2007).
- M.A.V.R. da Silva, M.d.D.M.C.R. da Silva, A.F.L.O.M. Santos, M.V. Roux, C. Foces-Foces, R. Notario, S.P. Verevkin, V.N. Emel?yanenko, R. Guzmán-Mejía y E. Juaristi, “Experimental and Computational Thermochemical Study of -Alanine (DL) and -Alanine”, J. Phys. Chem. B., 114, 16471-16480 (2010).
- E. Juaristi y R. Notario, “Computational Reexamination of the Eclipsed Conformation in cis-2-tert-Butyl-5-(tert-butylsulfonyl)-1,3dioxane”, Struct. Chem., 24, 1855-1862 (2013).
- E. Juaristi, “Obras 5. Análisis Conformacional y Modelado Molecular?, El Colegio Nacional: México (2014). ISBN: 978-607-724-083-9
- E. Juaristi y R. Notario, “Theoretical Examination of the S-C-P Anomeric Effect?, J. Org. Chem., 80, 2879-2883 (2015).
- E. Juaristi y R. Notario, "Theoretical Evidence for the Relevance of n(F)→ s*(C–X) (X = H, C, O, S) Stereoelectronic Interactions", J. Org. Chem., 81, 1192-1197 (2016).
- E. Juaristi, G. Gomes, A. Terent'ev, R. Notario y I. Alabugin, "Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular a-Effect", J. Am. Chem. Soc., 139, 10799-10813 (2017).
- E. Juaristi y R. Notario, "Theoretical Evidence for the Relevance of n(S)→s*(C–P), s(C–S) → s*(C–P), and n(F) → s*(C–X) (X = H, C, O, S) Stereoelectronic Interactions", en "Stereochemistry and Global Connectivity: The Legacy of Ernest Eliel", H.N. Cheng, Editor, ACS Symposium Series, Volumen 1258, American Chemical Society: Washington, D.C., 2017, Chapter 1, pp. 3-18.
- E. Juaristi y R. Notario, "Density Functional Theory Computation Reexamination of the Anomeric Effect in 2-Methoxy- and 2-Cyano-1,3-dioxanes and 1,3-Dithianes. Novel Stereoelectronic Interactions Involving the Cyano (C≡N:) Group Revealed by Natural Bond Order (NBO) Analysis", J. Org. Chem., 83, 10326-10333 (2018).
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One-Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One- Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- M.A. Ortega-Rojas, E. Castillo, R.S. Razo-Hernández, N. Pastor, E. Juaristi y J. Escalante, “Effect of the Substituent and Amino Group Position on the Lipase-Catalyzed Resolution of gamma-Amino Esters: A Molecular Docking Study Shedding Light on Candida antarctica lipase B Enantioselectivity”, Eur. J. Org. Chem. 4790-4802 (2021).
- L. Rivillas-Acevedo, R. Grande-Aztatzi, E. Juaristi, A. Vela y L. Quintanar, “Reversible Stereoisomer-Specific Cotton Effect of the Ligand Field Transitions at a Copper(II) Binding Site of the Prion Protein”, Eur. J. Inorg. Chem. 2022, e202100625 (2022).
Química Heterocíclica
El grupo de investigación del Dr. Juaristi ha trabajado durante más de 20 años en el estudio conformational y en la aplicación en síntesis orgánica de compuestos heterocíclicos conteniendo azufre vease, por ejemplo:
- E. Juaristi, “Conformational Analysis of Six-Membered, Sulfur-Containing Saturated Heterocycles”, Acc. Chem. Res., 22, 357-364 (1989).
- E. Juaristi, “1-Benzoyl-2(S)-tert-butyl-3-methylpyrimidinone”, en Encyclopedia of Reagents for Organic Synthesis, L.A. Paquette, ed., Wiley: New York, en prensa.
- E. Juaristi y M. Ordóñez, “Conformational Preference of the Sulfinyl Group in Six-Membered Heterocycles”, en Organosulfphur Chemistry, P. Page, ed., Academic Press: London, 1997].
- E. Juaristi, “1-Benzoyl-2(S)-tert-butyl-3-methylperhydropyrimidine-4-one”, en Encyclopedia of Reagents for Organic Synthesis, L.A. Paquette, Ed., Wiley, 2002.
- M.A. Iglesias-Arteaga, E. Castellanos y E. Juaristi, “Alternative Procedure for the Síntesis of Enantiopure 1-Benzoyl-2(S)-tert-butyl-3-methylperhydropyrimidin-4-one, a Useful Starting Material for the Enantioselective Synthesis of a-Substituted b-Amino Acids”, Tetrahedron: Asymmetry, 14, 577-580 (2003).
- I. Linzaga, J. Escalante, M. Muñoz y E. Juaristi, “NMR and X-Ray Crystallographic Studies of Axial and Equatorial 2-Ethoxy-2-oxo-1,4,2-oxazaphosphinane Derivatives”, Tetrahedron, 58, 8973-8978 (2002).
- M. Sosa-Rivadeneyra, L. Quintero, C. Anaya de Parrodi, S. Barnés, E. Castellanos y E. Juaristi, “Preparation and Diastereoselective Methylation of Enantiopure (S)-4-(1-Phenylethyl)-1,4-oxazin-2-ones”, Arkivoc (Número Especial dedicado a la Química Orgánica en México), 2003, 61-71 (2003).
- M.V. Roux, M.Temprado, P. Jiménez, J.Z. Dávalos, R. Notario, L. Garrido, R. Guzmán-Mejía y E. Juaristi, “Thermochemistry of 1,3-Dithiacyclohexane 1-Oxide (1,3-Dithiane Sulfoxide): A Calorimetric and Computational Study”, J. Org. Chem., 69, 5454-5459 (2004).
- A. Clara-Sosa, L. Pérez, M. Sánchez, R. Melgar-Fernández, E. Juaristi, L. Quintero y C. Anaya de Parrodi, “cis- and trans-N-(Benzylsulfinyl)hexa-hydrobenzoxazolidin-2-one as Novel Chiral Sulfinyl Transfer Reagents”, Tetrahedron, 60, 12147-12152 (2004).
- E. Navarro, F.J. González, P.D. Astudillo, M. Vázquez-Hernández, M. Hernández-Rodríguez y E. Juaristi, "The Role of Alkali and Alkaline Earth Metal Ions on the Hydrolysis of 2-Ferrocenyl-1,3-dioxane in Acetonitrile Solutions", Polish J. Chem., 81, 921-930 (2007).
- B.R. Díaz-Sánchez, M.A. Iglesias-Arteaga, R. Melgar-Fernández y E. Juaristi, "Synthesis of 2-Substituted-5-halo-2,3-dihydro-4(H)-pyrimidin-4-ones and Their Derivatization to Potential Precursors of α-Substituted β-Amino Acids Utilizing the Sonogashira Coupling Reaction", J. Org. Chem., 72, 4822-4825 (2007).
- A. R. Sting, D. Seebach, R. Melgar-Fernández y E. Juaristi,"(R,R)-2-t-Butyl-5-methyl-1,3-dioxolan-4-one: Update", en Electronic Encyclopedia of Reagents for Organic Synthesis, D. Crich, Editor; Wiley: New York, (2007); pp. 1-12.
- R. Melgar-Fernández, R.González-Olvera, J.L. Olivares-Romero, V.González-López, L. Romero-Ponce, M.Ramírez-Zárate, P. Demare, I. Regla y E.Juaristi “Synthesis of Novel Derivatives of(1S,4S)-2,5-Diazabicyclo[2.2.1]heptane and theirEvaluation as Potential Ligands in AsymmetricCatalysis”, Eur. J. Org. Chem., 655-672 (2008).
- R. González-Olvera, P. Demare, I. Regla y E. Juaristi, “Application of (1S,4S)-2,5-Diazabicyclo[2.2.1]heptaneDerivatives in Asymmetric Organocatalysis: theBiginelli Reaction”, Arkivoc, vi, 61-72 (2008).
- J.L. Olivares-Romero y E. Juaristi,“Synthesis of Two Novel Chiral Diamines Derivedfrom (S)-Proline and their Evaluation as Precursors of Diazaborolidines for the CatalyticBorane-Mediated Enantioselective Reduction ofProchiral Ketones”, Tetrahedron, 64,9992-9998 (2008).
- R. Melgar-Fernández, R. González-Olvera, J.Vargas-Caporali, R. Pérez-Isidoro y E. Juaristi,“Resolution of 5-Oxo-1-phenylpyrazolidine-3-carboxylicAcid and Synthesis of Novel Enantiopure AmideDerivatives”, Arkivoc, (viii), 55-75 (2010).
- H.A. Stefani, M.F.Z.J. Amaral, F.V. Singh,G. Reyes-Rangel y E. Juaristi,“Functionalization of (2S)-Isopropyl-5-iodo-2,3-dihydro-4(H)-pyrimidin-4-onesvia Suzuki-Miyaura Cross-Coupling Reaction UsingAryltrifluoroborate Salts: ConvenientEnantioselective Preparation of -Substituted--AminoAcids”, Eur. J. Org. Chem., 6393-6403 (2010).
- A. Tolomelli, G. Cardillo, L. Gentilucci, R. Juris, A. Viola y E. Juaristi, “Exploring the Reactivity of Alkyledene Malonamides: Synthesis of PolyfunctionalizedIsoxazolidinones, Aziridines, and Oxazolines”, Arkivoc, ?, 196-209(2012).
- J. Vargas-Caporali, C. Cruz-Hernández, G. Dewynter y E. Juaristi,”Synthesis of Versatile Bifunctional Derivatives of Chiral Diamines Obtained ThroughAnchimerically Assisted Nucleophilic Substitution Reactions on diasteromeric Phenylprolinols”, Heterocycles, 86, 1275-1300 (2012).
- A. Vega-Peñaloza, O. Sánchez-Antonio, M. Escudero-Casao, G. Tasnádi, F. Fulop y E. Juaristi, “Stereoselective Synthesis of Chiral Pyrrolidine Derivatives of (+)--Pinene Containing a -Amino Acid Moiety”, Synthesis, 45, 2458-2468 (2013).
- Y. Bandala, G. Reyes-Rangel, A. Obregón-Zúñiga, C. Cruz-Hernández, G. Corzo y E. Juaristi, “trans-Hexahydrobenzoxazolidinones in the Enantioselective Synthesis of 2-Amino Acids Containing Proteinogenic Side Chains”, Eur. J. Org. Chem., 2275-2283 (2014).
- E. Juaristi, “Obras 4. Síntesis Orgánica y Química Heterocíclica”, El Colegio Nacional: México (2014), ISBN 978-607-729-073-0
- S. Kumar, C. Cruz, S. Pal, R.K. Saunthwal, E. Juaristi y A.K. Verma, “An Expedient Tandem Approach to Benzothieno and Benzofuro-pyridines from o-Alkynyl Aldehydes via Silver-Catalyzed 6-endo-dig Ring Closure”, J. Org. Chem., 80, 10548-10560 (2015).
- L.A. Polindara-García y E. Juaristi, "Synthesis of Ugi-4CR and Passerini-3CR Adducts Under Mechanochemical Activation", Eur. J. Org. Chem., 2016, 1095-1102 (2016).
- G. Reyes-Rangel, J. Vargas-Caporali y E. Juaristi, "Asymmetric Michael Addition Reaction Organocatalyzed by Stereoisomeric Pyrrolidine Sulfinamides Under Neat Conditions. A Brief Study on Self-Disproportionation of Enantiomers", Tetrahedron, 73, 4707-4718 (2017).
- J. Vargas-Caporali y E. Juaristi, "Determination of Enantioselectivities by Mean of Chiral Stationary Phase HPLC in Order to Identify Effective Proline-Derived Organocatalysts", J. Brazilian Chem. Soc., 29, 896-915 (2018).
- A. Espinoza-Vázquez, F.J. Rodríguez-Gómez, E. Juaristi, M. Escudero-Casao, D. Angeles-Beltrán, G.E. Negrón-Silva y M. Palomar-Pardavé, "Triazoles Derived from ß-Amino Acids as Corrosion Inhibitors for AP1 5L X52 Steel Immersed in 1M HCl", Int. J. Electrochem. Sci., 13, 7517-7531 (2018).
- A. Espinoza-Vázquez, F.J. Rodríguez-Gómez, E. Juaristi, M. Escudero-Casao, D. Angeles-Beltrán, G.E. Negrón-Silva y M. Palomar-Pardavé, "Triazoles Derived from ß-Amino Acids as Corrosion Inhibitors for AP1 5L X52 Steel Immersed in 1M HCl", Int. J. Electrochem. Sci., 13, 7517-7531 (2018).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- O. Sánchez-Antonio, R. González-Olvera, A. Aguilera-Cruz, A. Reyes-Ramírez y E. Juaristi, "Diastereoselective Synthesis of New Isoindolone-Derived Fused Heterocycles and Their Crystallographic Structures", Tetrahedron, 75, 130594 (2019).
- L. Alvarez-Santamaría, E. Juaristi, A.B. Arroyo-Colín, J. Palma-Flores y J. Escalante, "Efficient Solvent-Free Preparation of Imines, and Their Subsequent Oxidation with m-CPBA to Afford Oxaziridines", Green and Sustainable Chem., 9, 143-154 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Synthesis of Diazaphosphol-2-oxides and Their Derivatives: Applications in Asymmetric Synthesis", en "Targets in Heterocyclic Systems", O.A. Attanasi, Editor, Italian Chemical Society, Volumen 23, Capítulo 16, pp 324-339 (2019).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- O. Sánchez-Antonio, R. González-Olvera, A. Aguilera-Cruz, A. Reyes-Ramírez y E. Juaristi, "Diastereoselective Synthesis of New Isoindolone-Derived Fused Heterocycles and Their Crystallographic Structures", Tetrahedron, 75, 130594 (2019).
- L. Alvarez-Santamaría, E. Juaristi, A.B. Arroyo-Colín, J. Palma-Flores y J. Escalante, "Efficient Solvent-Free Preparation of Imines, and Their Subsequent Oxidation with m-CPBA to Afford Oxaziridines", Green and Sustainable Chem., 9, 143-154 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Synthesis of Diazaphosphol-2-oxides and Their Derivatives: Applications in Asymmetric Synthesis", en "Targets in Heterocyclic Systems", O.A. Attanasi, Editor, Italian Chemical Society, Volumen 23, Capítulo 16, pp 324-339 (2019).
- M. Pérez-Venegas, M.N. Villanueva-Hernández, E. Peña-Cabrera y E. Juaristi, "Mechanochemically Activated Liebeskind-?Srogl (L-?S) Cross-?Coupling Reaction: Green Synthesis of meso-?Substituted BODIPYs", Organometallics, 39, 2561-2564 (2020).
- J.C. Jiménez-Cruz, R. Guzmán-Mejía, E. Juaristi, O. Sánchez-Antonio, M.A. García-Revilla, J.B. González-Campos y J. Aviña-Verduzco, "Preparation of aromatic ?-?hydroxyketones by means of Heck coupling of aryl halides and 2,?3-?dihydrofuran, catalyzed by a palladium(II) glycine complex under microwave irradiation", New Journal of Chemistry, 44, 13382-13392 (2020).
- O. Sánchez-Antonio, K.A. Romero-Sedglach, E.C. Vázquez-Orta y E. Juaristi, "New mesoporous silica-?supported organocatalysts based on (2S)?-?(1,?2,?4-?triazol-?3-?yl)?-?proline: efficient, reusable, and heterogeneous catalysts for the asymmetric aldol reaction", Molecules, 25, 4532 (2020).
- M. Pérez-Venegas, T. Arbeloa, J. Bañuelos, I. López-Arbeloa, N.E. Lozoya-Pérez, B. Franco, H.M. Mora-Montes, J.L. Belmonte-Vázquez, C.I. Bautista-Hernández, E. Peña-Cabrera, y E. Juaristi, “Mechanochemistry as a Sustainable Method for the Preparation of Fluorescent Ugi BODIPY Adducts”, Eur. J. Org. Chem., 253-265 (2021).
-
C. Cruz-Hernández y E. Juaristi, “On the Synthesis of a Novel Chiral Phosphorodiamidic Acid Containing the a-?Phenylethyl Moiety: Insights on its Conformation and Reactivity”, Synthesis, 54, 3801-3808 (2022).
-
I. J. Arroyo-Córdoba, G. Gamboa-Velázquez, C. G. Ávila-Ortiz, M. A. Leyva-Ramírez, M. T. Cortez-Picasso, M. A. García-Revilla, D. E. Ramírez-Ornelas, E. Peña-Cabrera y E. Juaristi, “Structure and Conformation of Novel BODIPY Ugi Adducts”, ChemistryOpen, 11, e202200197 (2022).
-
E. Juaristi, “What Is the Anomeric Effect?”, en “Oxygen, Stereoelectronic Effects”, Alabugin I, Kuhn, L, American Chemical Society, Washington, D.C., USA, las Secciones 3.8 y 3.9, que incluyen dos videos (2023).
Química Verde
- J.G. Hernández y E. Juaristi, “A Green Synthesis of ,- and ,-Dipeptides under Solvent-Free Conditions”, J. Org. Chem., 75, 7107-7111 (2010).
- J.G. Hernández y E. Juaristi, “Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Dipeptides: Improved Stereoinduction under Solvent-Free Conditions”, J. Org. Chem., 76, 1464-1467 (2011).
- J.G. Hernández y E. Juaristi, “Efficient Ball-Mill Procedure in the “Green” Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Dipeptides in the Presence of Water”, Tetrahedron, 67, 6953-6959 (2011).
- J.G. Hernández, Víctor García López y E. Juaristi, “Solvent-Free Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-containing Thiodipeptides under Ball-Milling Conditions”, Tetrahedron, 68, 92-97 (2012).
- J.G. Hernández y E. Juaristi, “Recent Efforts Directed to the Development of More Sustainable Asymmetric Organocatalysis”, Chem. Commun., (Feature Article), 48, 5396-5409 (2012).
- J.G. Hernández y E. Juaristi, “Reacciones Asimétricas Organocatalizadas en Ausencia de Disolvente: una Estrategia para Hacer mas “Verde” la Organocatálisis”, Educación Química, 24, 96-102 (2013).
- A. Vega-Peñaloza, O Sánchez-Antonio, C.G. Avila-Ortiz, M. Escudero-Casao y E. Juaristi, “An Alternative Synthesis of Chiral (S)-Proline Derivatives Containing a Thiohydantoin Moiety and Their Application in the Asymmetric Michael Addition Reaction Under Solvent-Free Conditions”, Asian J. Org. Chem., 3, 487-496 (2014).
- E. Machuca y E. Juaristi, “Asymmetric Organocatalytic Reactions Under Ball Milling”, en “Ball Milling Towards Green Synthesis: Applications, Projects, Challenges”, R. Brindaban y A. Stolle, Editors, Royal Society of Chemistry: Cambridge, UK, (2015); capítulo 4, pp. 81-95.
- C.G. Avila-Ortiz, M. López-Ortiz, A. Vega-Peñaloza, I. Regla y E. Juaristi, "Use of (R)-Mandelic Acid as Chiral Co-Catalyst in the Michael Addition Reaction Organocatalyzed by (1S,4S)-2-Tosyl-2,5-diazabicyclo[2.2.1]heptane under Solvent-Free Conditions", Asymmetric Catalysis, Topical Issue on Organocatalysis, 2, 37-44 (2015).
- L.A. Polindara-García y E. Juaristi, "Synthesis of Ugi-4CR and Passerini-3CR Adducts Under Mechanochemical Activation", Eur. J. Org. Chem., 1095-1102 (2016).
- J.M. Landeros y E. Juaristi, "Mechanochemical Synthesis of Dipeptides Using Mg-Al Hydrotalcite as Catalyst Under Solvent-Free Reaction Conditions", Eur. J. Org. Chem., 687-694 (2017).
- A. Obregón-Zúñiga, M. Guerrero-Robles y E. Juaristi, "Chiral Imidazolium Ionic Liquids Derived from (S)-Prolinamine as Organocatalysts in the Asymmetric Michael Reaction and Michael-Aldol Cascade Reaction under Solvent-Free Conditions", Eur. J. Org. Chem., 2692-2697 (2017).
- A. Obregón-Zúñiga y E. Juaristi, “(2S,4R)-Hyp-(S)-Phe-OMe Dipeptide Supported on Imidazolium Tagged Molecules as Recoverable Organocatalysts for Asymmetric Aldol Reactions Using Water as Reaction Medium", Tetrahedron, 73, 5373-5380 (2017).
- C. G. Avila-Ortiz, L. Díaz-Corona, E. Jiménez-González y E. Juaristi, "Asymmetric Michael Addition Organocatalyzed by a,ß-Dipeptides Under Solvent-Free Reaction Conditions", Molecules, 22, 1328-1342 (2017).
- A. Obregón Zúñiga y E. Juaristi, "Química Verde y sus Doce Principios: Rumbo a Procesos Sustentables", Conversus (Instituto Politécnico Nacional), No. 122, 4-5 (2016).
- M. Pérez-Venegas y E. Juaristi, "Mechanoenzymatic Resolution of Racemic Chiral Amines, A Green Technique for the Synthesis of Pharmaceutical Building Blocks", Tetrahedron, 74, 6453-6458 (2018).
- A. Fingerhut, J. Vargas-Caporali, M.A. Leyva-Ramírez, E. Juaristi y S.B. Tsogoeva, "Biominetic Non-Heme Iron(III) Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant", Molecules, 24, 3182 (2019).
- O. Sánchez-Antonio y E. Juaristi, "Synthesis of a New Chiral Organocatalyst Derived from (S)-Proline Containing a 1,2,4-Triazolyl Moiety and Its Application in the Asymmetric Aldol Reaction. Effect of Water", Tetrahedron Lett., 60, 151128 (2019).
- L. Alvarez-Santamaría, E. Juaristi, A.B. Arroyo-Colín, J. Palma-Flores y J. Escalante, "Efficient Solvent-Free Preparation of Imines, and Their Subsequent Oxidation with m-CPBA to Afford Oxaziridines", Green and Sustainable Chem., 9, 143-154 (2019).
- E. Juaristi, "Obras 8. Organocatálisis Asimétrica y Química Verde", El Colegio Nacional: México (2019). ISBN: 978-607-724-347-2.
- C.G. Avila-Ortiz, M. Pérez-Venegas, J. Vargas Caporali y E. Juaristi, "Recent Applications of Mechanochemistry in Enantioselective Synthesis", Tetrahedron Lett. Digest, 60, 1749-1757 (2019).
- A. Fingerhut, J. Vargas-Caporali, M.A. Leyva-Ramírez, E. Juaristi y S.B. Tsogoeva, "Biominetic Non-Heme Iron(III) Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant", Molecules, 24, 3182 (2019).
- O. Sánchez-Antonio y E. Juaristi, "Synthesis of a New Chiral Organocatalyst Derived from (S)-Proline Containing a 1,2,4-Triazolyl Moiety and Its Application in the Asymmetric Aldol Reaction. Effect of Water", Tetrahedron Lett., 60, 151128 (2019).
- L. Alvarez-Santamaría, E. Juaristi, A.B. Arroyo-Colín, J. Palma-Flores y J. Escalante, "Efficient Solvent-Free Preparation of Imines, and Their Subsequent Oxidation with m-CPBA to Afford Oxaziridines", Green and Sustainable Chem., 9, 143-154 (2019).
- E. Juaristi, "Obras 8. Organocatálisis Asimétrica y Química Verde", El Colegio Nacional: México (2019). ISBN: 978-607-724-347-2.
- C.G. Avila-Ortiz, M. Pérez-Venegas, J. Vargas Caporali y E. Juaristi, "Recent Applications of Mechanochemistry in Enantioselective Synthesis", Tetrahedron Lett. Digest, 60, 1749-1757 (2019).
- M. Pérez-Venegas y E. Juaristi, "Mechanochemical and Mechanoenzymatic Synthesis of Pharmacologically Active Compounds: A Green Perspective", ACS Sustainable Chemistry & Engineering, 8, 8881-8893 (2020).
- C.G. Avila-Ortiz y E. Juaristi, "Novel methodologies for chemical activation in organic synthesis under solvent-?free reaction conditions", Molecules, 25, 3579 (2020).
- M. Anselmi, P. Stavole, E. Boanini, A. Bigi, E. Juaristi y L. Gentilucci, "Solvent-Free and Minimal Solvent-Assisted Peptide Bond Formation Promoted by Nanocrystalline Hydroxyapatite", Future Medicinal Chem., 12, 479-491 (2020).
- M. Pérez-Venegas, M.N. Villanueva-Hernández, E. Peña-Cabrera y E. Juaristi, "Mechanochemically Activated Liebeskind-?Srogl (L-?S) Cross-?Coupling Reaction: Green Synthesis of meso-?Substituted BODIPYs", Organometallics, 39, 2561-2564 (2020).
- O. Sánchez-Antonio, K.A. Romero-Sedglach, E.C. Vázquez-Orta y E. Juaristi, "New mesoporous silica-?supported organocatalysts based on (2S)?-?(1,?2,?4-?triazol-?3-?yl)?-?proline: efficient, reusable, and heterogeneous catalysts for the asymmetric aldol reaction", Molecules, 25, 4532 (2020).
- A. Obregón-Zúñiga y E. Juaristi, "Ionic Liquids: Design and Application", en "Green Chemistry in Drug Discovery: From Academia to Industry", P.F. Richardson, Editor, Springer Nature (2021), Capítulo 6, pp. 179-210.
- G. Gamboa-Velázquez y E. Juaristi, “Mechanoenzymology in the Kinetic Resolution of ß-?Blockers: Propranolol as a Case Study”, ACS Organic & Inorganic Au, 2, 343-350 (2022).
- E. Juaristi y C. G. Ávila-Ortiz, "Salient Achievements in Synthetic Organic Chemistry Enabled by Mechanochemical Activation", Synthesis, 55, 2439-2459 (2023).
Resonancia Magnética Nuclear
Principalmente sus aplicaciones en la determinación de la conformación preferida de compuestos heterocíclicos. Por ejemplo:
- E. Juaristi, J. Guzmán, V.V. Kane y R.S. Glass, “1H and 13C NMR Studies of the Mono-S-oxides of 1,2-, 1,3-, and 1,4-Dithianes”, Tetrahedron, 40, 1477-1485 (1984).
- B.M. Pinto, D.B. Johnston, R. Nagelkerke, E. Juaristi y E.A. González, “Determination of the Nitrogen Lone Pair Orientation of 5-methyl-5-aza-1,3-dithiacyclohexane”, Can. J. Chem., 67, 2067-2070 (1989).
- E. Juaristi, G. Cuevas y A. Vela, “Stereoelectronic Interpretation for the Anomalous 1H NMR Chemical Shifts and One-Bond C-H Coupling Constants”, J. Am. Chem. Soc., 116, 5796-5804 (1994).
- C. Anaya de Parrodi, G.E. Moreno, L. Quintero y E. Juaristi, “Application of Phosphorylated Reagents in the Determination of the Optical Purity of Chiral Alcohols”, Tetrahedron: Asymmetry, 9, 2093-2099 (1998).
- I. Linzaga, J. Escalante, M. Muñoz y E. Juaristi, “NMR and X-Ray Crystallographic Studies of Axial and Equatorial 2-Ethoxy-2-oxo-1,4,2-oxazaphosphinane Derivatives”, Tetrahedron, 58, 8873 - 8978 (2002).
- G. Cuevas y E. Juaristi, “Manifestation of Stereoelectronic Effects on the Calculated Carbon-Hydrogen Bond Lengths and 1JC-H NMR Coupling Constants in Cyclohexane, Six-Membered Heterocycles, and Cyclohexanone Derivatives”, J. Am. Chem. Soc., 124, 13088-13096 (2002).
- I. Linzaga, J. Escalante, M. Muñoz y E. Juaristi, “NMR and X-Ray Crystallographic Studies of Axial and Equatorial 2-Ethoxy-2-oxo-1,4,2-oxazaphosphinane Derivatives”, Tetrahedron, 58, 8973-8978 (2002).
- G.E. Moreno, V.M. Mastranzo, L. Quintero, C. Anaya de Parrodi y E. Juaristi, “The Use of N,N’Di[a-Phenylethyl]ethane-1,2-diamines as Phosphorylated Chiral Derivatizing Agents for the Determination of the Enantiomeric Purity of Chiral Secondary Alcohols”, Rev. Soc. Quím. Méx., (Número especial dedicado a A. Romo de Vivar), 47, 127-129 (2003).
- eK. Martínez-Mayorga, E. Juaristi y G. Cuevas, “Manifestation of Stereoelectronic Effects on the Calculated Carbon-Hydrogen Bond Lengths and One Bond 1JC-H NMR Coupling Constants Relative Aceptor Ability of the Carbonyl (C=O), Thiocarbonyl (C=S), and Methyledene (C=CH2) Groups Towards C-H Donor Bonds”, J. Org. Chem., 69, 7266-7276 (2004).
- A. Ariza Castolo, V. Bakhmutov, R. Contreras, N. Farfán, A. Flores, B. Gordillo, E. Juaristi, A. Paz, M. J. Rosales y R. L. Santillán, "Ejemplos Prácticos del Uso de la Resonancia Magnética Nuclear en la Química. Lecturas para Estudiantes", Cinvestav-IPN: México (2006), ISBN: 968-9020-00-5.
- M. Hernández-Rodríguez y E. Juaristi, "Structurally Simple Chiral Thioureas as Chiral Solvating Agents in the Enantiodiscrimination of Carboxylic Acids", Tetrahedron, 63, 7673-7678 (2007).
- G. Huelgas, S. Bernés, M. Sánchez, L. Quintero, E. Juaristi, C. Anaya de Parrodi y P. J. Walsh, "Synthesis and Dynamics of Atropisomeric (S)-N-(α-Phenylelthyl)benzamides", Tetrahedron, 63, 12655-12664 (2007).
- E. Juaristi y G. Cuevas, "Manifestations of Stereoelectronic Interactions in 1JC-H One Bond Coupling Constants", Acc. Chem. Res., 40, 961-970 (2007).
- E. Jiménez-González, C.G. Avila-Ortiz, R. González-Olvera, J. Vargas-Caporali, G. Dewynter y E. Juaristi, “Synthesis in Solution of Novel Seven-Membered Cyclic Dipeptides Containing - and -Amino Acids”, Tetrahedron, 68, 9842-9852 (2012).
- E. Juaristi, "Obras 7. Reacciones Estereoselectivas y Aplicaciones de la Resonancia Magnética Nuclear", El Colegio Nacional: México (2015). ISBN: 978-607-724-110-2.
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One- Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One-Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One-Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- I. J. Arroyo-Córdoba, G. Gamboa-Velázquez, C. G. Ávila-Ortiz, M. A. Leyva-Ramírez, M. T. Cortez-Picasso, M. A. García-Revilla, D. E. Ramírez-Ornelas, E. Peña-Cabrera y E. Juaristi, “Structure and Conformation of Novel BODIPY Ugi Adducts”, ChemistryOpen, 11, e202200197 (2022).
Síntesis asimétrica
Pionero en México del desarrollo de la síntesis asimétrica. Publicaciones representativas:
- E. Juaristi, ed., “Enantioselective Synthesis of b-Amino Acids”, Wiley-VCH: New York, 1997,
- E. Juaristi y H. López-Ruiz, “Recent Advances in the Enantioselective Synthesis of b-Amino Acids”, Current Medicinal Chemistry, 6, 983-1004 (1999),
- “Recent Applications of a-Phenylethylamine in the Preparation of Enantiopure Compounds”, Tetrahedron: Asymmetry, 10, 2441-2495 (1999).
- J.L. León-Romo, C.I. Virués, J. Aviña, I. Regla y E. Juaristi, “Preparation of (R)- and (S)-a-Methyldopa from a Chiral Hydantoin Containing the a-Phenethyl Group”, Chirality, 14, 144 (2002).
- V.M. Gutiérrez-García, G. Reyes-Ragel, O. Muñoz-Muñiz y E. Juaristi, “Enantioselective Síntesis of b-Amino Acids. Part 13. Diastereoselective Alkylation of Dianions Derived from Chiral Analogs of b-Aminopropionic Acid Containing the a-Phenylethyl Group”, Helv. Chim. Acta, 85, 4189-4199 (2002).
- V.M. Mastranzo, L. Quintero, C. Anaya de Parrodi, E. Juaristi y P.J. Walsh, “Use of Diamines Containing the a-Phenethyl Group as Chiral Ligands in the Asymmetric Hydrosilylation of Prochiral Ketones”, Tetrahedron, 60, 1781-1789 (2004).
- E. Castellanos, G. Reyes-Rangel y E. Juaristi, “Diastereoselective Electrophilic Amination of Chiral 1-Benzoyl-2-isopropyl-3-methyl-perhydropyrimidin-4-one in the Asymmetric Synthesis of a-Substituted a,b-Diaminopropionic Acid”, Helv. Chim. Acta, 87, 1016-1024 (2004).
- A. Clara-Sosa, L. Pérez, M. Sánchez, R. Melgar-Fernández, E. Juaristi, L. Quintero y C. Anaya de Parrodi, “cis- and trans-N-(Benzylsulfinyl)-hexahydrobenzoxazolidin-2-one as Novel Chiral Sulfinyl Transfer Reagents”, Tetrahedron, 60, 12147-12152 (2004).
- E. Juaristi y V. Soloshonok, Eds., “Second Edition of Enantioselective Synthesis of b-Amino Acids:”, Wiley: New York, ISBN: 0-471-46738-3 (2005).
- G. Reyes-Rangel, V. Marañón, C.G. Avila-Ortiz, C. Anaya, L. Quintero y E. Juaristi, "Enantioselective Synthesis of (R)-2-Amino-3-phosphonopropionic Acid, (R)-AP-3, Via Diastereoselective Azidation of (4S,5R)-trans-N-Diethoxyphosphoryl)-propionyhexahydrobenzoxazolidin-2-one", Tetrahedron, 62, 8404-8409 (2006).
- V.M. Mastranzo, E. Santacruz, G. Huelgas, E. Paz, M.V. Sosa-Rivadeneyra, S. Bernes, E. Juaristi, L. Quintero y C. Anaya de Parrodi, "Synthesis of Novel Chiral Ligands Containing the N-(S)-alpha-Phenylethyl Group and Their Evaluation as Activators in the Enantioselective Addition of Et2Zn to Benzaldehyde", Tetrahedron: Asymmetry, 17, 1663-1670 (2006).
- F. García-Flores, L. S. Flores-Michel y E. Juaristi, "Asymmetric Allylation of Benzoyl-hydrazones Promoted by Novel C2-Symmetric Bis-Sulfoxide Organocatalysts", Tetrahedron Lett., 47, 8235-8238 (2006).
- Y. Liu, R. Melgar-Fernández y E. Juaristi, "Enantioselective Amination of alpha-Phenyl alpha-Cyanoacetate Catalyzed by Chiral Amines Incorporating the alpha-Phenylethyl Auxiliary", J. Org. Chem., 72, 1522-1525 (2007).
- R. Guzmán-Mejía, G. Reyes-Rangel y E. Juaristi, "Preparation of Chiral Derivatives of β-Alanine Containing the α-Phenylethyl Group: Useful Starting Materials for the Asymmetric Synthesis ofβ-Amino Acids", Nature Protocols, 2, 2759-2766 (2007).
- E. Juaristi, B.R.Díaz y J.L. Olivares,“Dioxa-, Oxathia- and Dithiazines”, en Comprehensive HeterocyclicChemistry III, Oxford, (2007); capítulo 9.10, pp.
- B.R. Díaz-Sánchez, M.A. Iglesias-Arteaga, R. Melgar-Fernández y E. Juaristi,“Synthesis of 2-Substituted-5-halo-2,3-dihydro-4(H)-pyrimidin-4-ones and Their Derivatization to Potential Precursors of -Substituted -Amino Acids Utilizing the Sonogashira Coupling Reaction” J. Org. Chem., 72, 4822-4825 (2007).
- R. Melgar-Fernández, R. González-Olvera, J.L. Olivares-Romero, V. González-López, L. Romero-Ponce, M. Ramírez-Zárate, P. Demare, I. Regla y E. Juaristi “Synthesis of Novel Derivatives of (1S,4S)-2,5-Diazabicyclo[2.2.1]heptane and their Evaluation as Potential Ligands in Asymmetric Catalysis”, Eur. J. Org. Chem., 655-672 (2008).
- R. González-Olvera, P. Demare, I. Regla y E. Juaristi,“Application of (1S,4S)-2,5-Diazabicyclo[2.2.1]heptane Derivatives in Asymmetric Organocatlysis: the Biginelli Reaction”, Arkivoc, Número Especial de Homenaje al Prof. Torbjorn Norin, vi, 61-72 (2008).
- M. Hernández-Rodríguez, C.G. Avila-Ortiz, J. Martín del Campo, D. Hernández-Romero, M.J. Rosales-Hoz y E. Juaristi, “Synthesis of Novel Chiral (Thio) Ureas and their Application as Organocatalysts in Asymmetric Synthesis”, Australian J. Chem., 61, 364-375 (2008).
- J.L. Olivares-Romero y E. Juaristi,“Synthesis of Two Novel Chiral Diamines Derived from (S)-Proline and their Evaluation as Precursors of Diazaborolidines for the Catalytic Borane-Mediated Enantioselective Reduction of Prochiral Ketones”, Tetrahedron, 64, 9992-9998 (2008).
- G. Reyes-Rangel, E. Jiménez-González, J.L. Olivares-Romero y E. Juaristi,“Enantioselective Synthesis of -Amino Acids Using Hexahydrobenzoxazolidi-nones as Chiral Auxiliaries”, Tetrahedron: Asymmetry, 2839 - 2849 (2008)
- Y. Bandala, R. Melgar-Fernández, R. Guzmán-Mejía, J.L. Olivares-Romero, B.R. Díaz-Sánchez, R. González-Olvera y E. Juaristi, “Optimized Methodologies in Asymmetric Organic Synthesis Applying Microwaves”, J. Mex. Chem. Soc., 53, 146-153 (2009).
- E. Forró, L. Schoenstein, L. Kiss, A. Vega-Peñaloza, E. Juaristi y F. Fulop, “Direct Enzymatic Route for the Preparation of Novel Enantiomerically Enriched Hydroxylated -Amino Ester Stereoisomers”, Molecules, 15, 3998-4010 (2010).
- H.A. Stefani, M.F.Z.J. Amaral, F.V. Singh, G. Reyes-Rangel y E. Juaristi, “Functionalization of (2S)-Isopropyl-5-iodo-2,3-dihydro-4(H)-pyrimidin-4-ones via Suzuki-Miyaura Cross-Coupling Reaction Using Aryltrifluoroborate Salts: Convenient Enantioselective Preparation of -Substituted--Amino Acids”, Eur. J. Org. Chem., 6393-6403 (2010).
- Y. Bandala y E. Juaristi, “Recent Advances in the Application of -Phenylethylamine (-PEA) in the Preparation of Enantiopure Compounds”, Aldrichim. Acta, 43, 65-78 (2010).
- J.G. Hernández y E. Juaristi, “Efficient Ball-Mill Procedure in the “Green” Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Dipeptides in the Presence of Water”, Tetrahedron, 67, 6953-6959 (2011).
- M. Escudero-Casao y E. Juaristi, “Asymmetric Synthesis of 2-homo-tert-leucine mediated via Radical Addition to Enantiopure N-Fumaroyl-hexahydrobenzo-2-oxazolidinone”, Helv. Chim. Acta, 95, 1714-1722 (2012).
- J.G. Hernández y E. Juaristi, “Recent Efforts Directed to the Development of More Sustainable Asymmetric Organocatalysis”, Chem. Commun., (Feature Article), 48, 5396-5409 (2012).
- G. Reyes-Rangel, Y. Bandala y E. Juaristi, “Asymmetric Allylation of -Ketoester-Derived N-Benzoylhidrazones Promoted by a Chiral C2-Symmetric bis-Sulfoxide/N-Oxide Lewis Base: Highly Enantioselective Synthesis of Quaternary -Substituted -Allyl--Amino Acids”, Chirality, 25, 529-540 (2013).
- E. Juaristi, “Obras 2, Síntesis Asimétrica”, El Colegio Nacional: México (2013), ISBN 978-607-724-027-3.
- Y. Bandala, G. Reyes-Rangel, A. Obregón-Zúñiga, C. Cruz-Hernández, G. Corzo y E. Juaristi, “trans-Hexahydrobenzoxazolidinones in the Enantioselective Synthesis of 2-Amino Acids Containing Proteinogenic Side Chains”, Eur. J. Org. Chem., 2275-2283 (2014).
- E. Machuca y E. Juaristi, “Asymmetric Organocatalytic Reactions Under Ball Milling”, en “Ball Milling Towards Green Synthesis: Applications, Projects, Challenges”, R. Brindaban y A. Stolle, Editors, Royal Society of Chemistry: Cambridge, UK, (2015); capítulo 4, pp. 81-95.
- G. Reyes-Rangel, J. Vargas-Caporali y E. Juaristi, "In Search of Diamine Analogs of the a,a-Diphenylprolinol Priviledged Chiral Organocatalyst. Synthesis of Diamine Derivatives of ,-Diphenyl-(S)-prolinol and Their Application as Organocatalysts in the Asymmetric Michael and Mannnich Reactions", Tetrahedron, 72, 379-391 (2016).
- J. Vargas-Caporali y E. Juaristi, "The Development of Chiral Phase Chromatography in Connection with the Enantioselective Synthesis of ß-Amino Acids", Isr. J. Chem., 57, 896-912 (2017).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatalysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Synthesis of Diazaphosphol-2-oxides and Their Derivatives: Applications in Asymmetric Synthesis", en "Targets in Heterocyclic Systems", O.A. Attanasi, Editor, Italian Chemical Society, Volumen 23, Capítulo 16, pp 324-339 (2019).
- C.G. Avila-Ortiz, M. Pérez-Venegas, J. Vargas Caporali y E. Juaristi, "Recent Applications of Mechanochemistry in Enantioselective Synthesis", Tetrahedron Lett. Digest, 60, 1749-1757 (2019).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Multifunctional Phosphoramide-(S)-prolinamide Derivatives as Efficient Organocatalysts in Asymmetric Aldol and Michael Reactions", New J. Chem., 43, 5455-5465 (2019).
- C. Cruz-Hernández, J.M. Landeros y E. Juaristi, "Synthesis of Diazaphosphol-2-oxides and Their Derivatives: Applications in Asymmetric Synthesis", en "Targets in Heterocyclic Systems", O.A. Attanasi, Editor, Italian Chemical Society, Volumen 23, Capítulo 16, pp 324-339 (2019).
- C.G. Avila-Ortiz, M. Pérez-Venegas, J. Vargas Caporali y E. Juaristi, "Recent Applications of Mechanochemistry in Enantioselective Synthesis", Tetrahedron Lett. Digest, 60, 1749-1757 (2019).
- M. Pérez-Venegas y E. Juaristi, "Mechanochemical and Mechanoenzymatic Synthesis of Pharmacologically Active Compounds: A Green Perspective", ACS Sustainable Chemistry & Engineering, 8, 8881-8893 (2020).
- E. Juaristi, “Recent developments in next generation (S)-proline-derived chiral organocatalysts”, Tetrahedron, 88, 132143 (2021).
-
Síntesis de Compuestos con Actividad Biólogica
Principalmente en el área de la síntesis enantioselectiva de a- y b-amino ácidos. Por ejemplo:
- El a-amino-b-hidroxiácido 9-Bmt presente en el immunosupresor ciclosporina [D. Seebach, E. Juaristi, et al., Helv. Chim. Acta, 70, 237-261 (1987)].
- El ácido (S)-2-amino-fosfonopentanoico utilizado en pacientes con el mal de Alzheimer [O. García-Barradas y E. Juaristi, Tetrahedron, 51, 3423-3434 (1995)].
- La preparación del a-hidroxi-b-amino ácido presente en el compuesto anticarcinogénico taxol [J. Escalante y E. Juaristi, Tetrahedron Lett., 36, 4397-4400 (1995)].
- La síntesis enantioselectiva del (R)- y (S)-AP6, potentes agonistas del receptor AMPA [Tetrahedron Asymmetry, 8, 1511-1514 (1997)] y La preparación de la (S)-a-metildopa, útil en el tratamiento del Parkinson [J.L. León-Romo, C.I. Virués, J. Aviña, I. Regla y E. Juaristi, Chirality, 14, 144 (2002)].
- R. Melgar-Fernández, P. Demare, E. Hong, J. Escalante, O. Muñoz-Muñiz, E. Juaristi y I. Regla, “Síntesis and Cardiovascular Activity of Metoprolol Analogs”, Bioorg. Med. Chem. Lett., 14, 191-194 (2004).
- G. Reyes-Rangel, V. Marañón, C.G. Avila-Ortiz, C. Anaya, L. Quintero y E. Juaristi, "Enantioselective Synthesis of (R)-2-Amino-3-phosphonopropionic Acid, (R)-AP-3, Via Diastereoselective Azidation of (4S,5R)-trans-N-Diethoxyphosphoryl)-propionylhexahydrobenzoxazolidin-2-one", Tetrahedron, 62, 8404-8409 (2006).
- P. Zubrzak, H. Williams, G.M. Coast, R.E. Isaac, G. Reyes-Rangel, E. Juaristi, J. Zabrocki y R.J. Nachman, "-Amino Acid Analogs of an Insect Neuropeptide Feature Potent Bioactivity and Resistance to Peptidase Hydrolysis", Biopolymers, Peptide Science, 88, 76-82 (2007).
- E. Juaristi, "Retos para el Desarrollo de Fármacos en México", en"Diseño y Producción de Fármacos", E. Juaristi, Coordinador, El Colegio Nacional: en México (2007), capítulo 1, pp. 4-18.
- E. Juaristi, Coordinador, "Diseño y Producción de Fármacos", El Colegio Nacional: México (2007). ISBN 978-970-640-356-8.
- S. Taneja-Bageshwar, A. Strey, P. Zubrzak, H. Williams, G. Reyes-Rangel, E. Juaristi, P. Pietrantonio y R.J. Nachman,“Identification of Selective and Non-Selective, Biostable -Amino Acid Agonists of Recombinant Insect Kinin Receptors from the Southern Cattle Tick Boophilus Microplus and Mosquito Aedes Aegypti”, Peptides, 29, 302-309 (2008).
- R.J. Nachman, O.B. Aziz, M. Davidovitch P. Zubrzak, R.E. Isaac, A. Strey, G. Reyes-Rangel, E. Juaristi, H.J.Williams y M. Altstein, “Biostable -Amino Acid PK/PBAN Analogs: Agonist and Antagonist Properties”, Peptides, 30, 608-615 (2009).
- L. Gentilucci, G. Cardillo, A. Tolomelli, R. De Marco, A. Garelli, S. Spampinato, A. Sparta y E. Juaristi, “Synthesis and Conformational Analysis of Cyclotetrapeptide Mimetic -Turn Templates, and Validation as 3D Scaffolds”, ChemMedChem, 4, 517-523 (2009).
- E. Garay, G. Patiño-López, S. Islas, L. Alarcón, E. Canche-Pool, R. Valle, O. Medina, G.C. Granados, B.C. Munguía, E. Juaristi, V. Ortiz-Navarrete y L. González-Mariscal, “CRTAM, a Molecule Involved in Epithelial Cell Adhesion”, J. Cell. Biochem., 111, 111-122 (2010).
- J. Rocha, V. Flores, R. Cabrera, A. Soto-Guzmán, G. Granados, E. Juaristi, G. Guarneros y M. de la Torre, “Evolution and Some Functions of the NprR-NprRB Quorum-Sensing System in the Bacillus-Cerens Group”, Appl. Microbiol. Biotechnol., 94, 1069-1078 (2012).
- A. Tolomelli, M. Baiula, L. Belvisi, A. Viola, L. Gentilucci, S. Troisi, s.D. Dattoli, S. Spampinato, M. Civera, E. Juaristi y M. Escudero, “Modulation of ?3- and 51- Integrin Mediated Adhesion by Dehydro- -amino Acids Containing Peptidomimetics”, European Journal of Medicinal Chemistry, 66, 258-268 (2013).
- A. Greco, R. de Marco, S. Tani, D. Giacomini, P. Galletti, A. Tolomelli, E. Juaristi y L. Gentilucci, “Convenient Synthesis of the Antibiotic Linezolid via a 1,3-Oxazolidin-2,4-dione Intermediate Derived from the Chiral Building Block Isoserine”, Eur. J. Org. Chem., pp 7614-7620, 2014.
- E. Juaristi, “Obras 6. Síntesis de Aminoácidos, Péptidos y Otros Compuestos con Actividad Biológica”, El Colegio Nacional: México (2014). ISBN: 978-607-724-096-9
- A. Tolomelli, M. Baiula, A. Viola, L. Ferrazano, L. Gentilucci, S.D. Datolli, S. Spampinato, E. Juaristi y M. Escudero, "Dehydro--proline Containing Peptidominetics as Selective 41 Integrin Antagonists: Stereochemical Recognition in Lignan-Receptor Interaction", ACS Medicinal Chemistry Letters, 6, 701-706 (2015).
- R. De Marco, A. Tolomelli, E. Juaristi y L. Gentilucci, "Integrin Ligands with a/ß-Hybrid Peptide Structure: Design, Bioactivity and Conformational Aspects", Medicinal Research Reviews, 36, 389-424 (2016).
- M. Pérez-Venegas y E. Juaristi, "Mechanoenzymatic Resolution of Racemic Chiral Amines, A Green Technique for the Synthesis of Pharmaceutical Building Blocks", Tetrahedron, 74, 6453-6458 (2018).
- M. Pérez-Venegas y E. Juaristi, "Mechanochemical and Mechanoenzymatic Synthesis of Pharmacologically Active Compounds: A Green Perspective", ACS Sustainable Chemistry & Engineering, 8, 8881-8893 (2020).
- M. Pérez-Venegas, A.M. Rodríguez-Treviño y E. Juaristi, “Dual Mechanoenzymatic Kinetic Resolution of (±)?-?Ketorolac", ChemCatChem, 12, 1782-1788 (2020).
- J.R. Valdez-Camacho, Y. Pérez-Salgado, A. Espinoza-Guillén, V. Gómez-Vidales, C.A. Tavira-Montalván, A. Meneses-Acosta, M.A. Leyva, M.G. Vázquez-Ríos, E. Juaristi,H. Hoepfl y J. Escalante, "Synthesis, structural characterization and antiproliferative activity on MCF-?7 and A549 tumor cell lines of [Cu(N-?N)?(ß3-?aminoacidate)?]?NO3 complexes (Casiopeínas)", Inorganica Chimica Acta, 506, 119542 (2020).
- G. Gamboa-Velázquez y E. Juaristi, “Mechanoenzymology in the Kinetic Resolution of ß-?Blockers: Propranolol as a Case Study”, ACS Organic & Inorganic Au, 2, 343-350 (2022).
Síntesis de péptidos no naturales
Se ha consolidado un laboratorio de síntesis de péptidos. Algunas publicaciones representativas son:
- P. Zubrzak, H. Williams, G.M. Coast, R.E. Isaac, G. Reyes-Rangel, E. Juaristi, J. Zabrocki y R.J. Nachman, “-Amino Acid Analogs of an Insect Neuropeptide Feature Potent Bioactivity and Resistance to Peptidase Hydrolysis”, Biopolymers, Peptide Science, 88, 76-82 (2007).
- Y. Bandala, I.A. Rivero, T. González, J. Aviña, y E. Juaristi, “Solid Phase Synthesis of Novel /-Tetrapeptides and Electrospray Ionization Mass Spectrometric Evaluation of Their Metal Cation Complexation Behavior”, J. Phys. Org. Chem., 21, 349-358 (2008).
- S. Taneja-Bageshwar, A. Strey, P. Zubrzak, H. Williams, G. Reyes-Rangel, E. Juaristi, P. Pietrantonio y R.J. Nachman, “Identification of Selective and Non-Selective, Biostable -Amino Acid Agonists of Recombinant Insect Kinin Receptors from the Southern Cattle Tick Boophilus Microplus and Mosquito Aedes Aegypti”, Peptides, 29, 302-309 (2008).
- R.J. Nachman, O.B. Aziz, M. Davidovitch P. Zubrzak, R.E. Isaac, A. Strey, G. Reyes-Rangel, E. Juaristi, H.J.Williams y M. Altstein, “Biostable -Amino Acid PK/PBAN Analogs: Agonist and Antagonist Properties”, Peptides, 30, 608-615 (2009).
- J.G. Hernández y E. Juaristi, “A GreenSysnthesis of ,-and ,-Dipeptidesunder Solvent-Free Conditions”, J. Org. Chem.,75, 7107-7111 (2010).
- Y. Bandala y E. Juaristi, “Applications of -Peptidesin Chemistry, Biology and Medicine”, en“Festchrift in Honor of Prof. Dr. LeopoldoGarcía-Colin 80th Birthday”, A. Macias y L.Dagdug, Eds., World Scientific: Singapore(2010); capítulo 12, pp. 183-198.
- E. Jiménez-González, C.G. Avila-Ortiz, R. González-Olvera, J. Vargas-Caporali, G. Dewynter y E. Juaristi, “Synthesis in Solution of Novel Seven-Membered Cyclic Dipeptides Containing - and -Amino Acids”, Tetrahedron, 68, 9842-9852 (2012).
- E. Juaristi, “Obras 6. Síntesis de Aminoácidos, Péptidos y Otros Compuestos con Actividad Biológica”, El Colegio Nacional: México (2014). ISBN: 978-607-724-096-9.
- E. Machuca, Y. Rojas y E. Juaristi, “Synthesis and Evaluation of (S)-Proline-Containing ,-Dipeptides as Organocatalysts in Solvent-Free Asymmetric Aldol Reactions Under Ball-Milling Conditions”, Asian J. Org. Chem., 4, 46-53 (2015).
- A. Obregón-Zúñiga y E. Juaristi, (2S,4R)-Hyp-(S)-Phe-OMe Dipeptide Supported on Imidazolium Tagged Molecules as Recoverable Organocatalysts for Asymmetric Aldol Reactions Using Water as Reaction Medium", Tetrahedron, 73, 5373-5380 (2017).
- M. Pérez-Venegas, G. Reyes-Rangel, A. Neri, J. Escalante y E. Juaristi, "Mechanochemical Enzymatic Resolution of N-Benzylated-ß3-Amino Esters", Beilstein J. Org. Chem., 13, 1728-1734 (2017).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatalysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatlysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- C. Cruz-Hernández, P.E. Hernández-González y E. Juaristi, "Proline-Glycine Dipeptidic Derivatives of Chiral Phosphoramides as Organocatlysts for the Enantiodivergent Aldol Reaction of Arylaldehydes and Isatins with Cyclohexanone in the Presence of Water", Synthesis, 50, 3445-3459 (2018).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- J.M. Landeros, L. Suchy, C.G. Avila-Ortiz, N. Maulide y E. Juaristi, "Dendrimeric a,ß-Dipeptidic Conjugates as Organocatalysts in Asymmetric Michael Addition Reaction of Isobutyraldehyde to N-Phenyl-Maleimides", Monatsch. Chem., 150, 777-788 (2019).
- J.M. Landeros, C. Cruz-Hernández y E. Juaristi, “a-Amino Acids and a,b-Dipeptides Intercalated into Hydrotalcite: Efficient Catalysts in the Asymmetric Michael Addition Reaction of Aldehydes to N-Substituted Maleimides”, Eur. J. Org. Chem. 5117-5126 (2021).
Síntesis Orgánica
Hemos desarrollado metodologías y reactivos de utilidad en síntesis orgánica; por ejemplo:
- E. Juaristi, “1-Benzoyl-2(S)-tert-butyl-3-methylperhydropyrimidin-4-one”, en Encyclopedia of Reagents for Organic Synthesis L.A. Paquette, ed., Wiley: Chichester, en prensa.
- J. Escalante, M.A. González-Tototzin, J. Aviña, O. Múñoz-Múñiz y E. Juaristi, “Synthesis of b-Lactams and Cyclo-b-dipeptides from b-Amino Acids”, Tetrahedron57, 1883-1890 (2001).
- Reyes y E. Juaristi, “Convenient Route for the Preparation of C2-Symmetric 2,3-Diphenylaziridine”, Chirality, 10, 95-99 (1998). (4) C.A. de Parrodi, E. Juaristi, L. Quintero y A. Clara-Sosa, “A Useful Route to Benzoxazolidin-2-ones”, Tetrahedron: Asymmetry, 8, 1075-1082 (1997).
- M.A. Iglesias-Arteaga, C. G. Avila-Ortiz y E. Juaristi, “Tandem Reactions Initiated by the Oxidative Decarboxylation of 1-Benzoyl-2(S)-tert-butyl-6(S)-carboxy-perhydropyridimidin-4-one”, Tetrahedron Lett., 43, 5297 (2002).
- M.A. Iglesias-Arteaga, E. Castellanos y E. Juaristi, “Alternative Procedure for the Synthesis of Enantiopure 1-Benzoyl-2(S)-tert-butyl-3-methylperhydropyrimidin-4-one, a Useful Starting Material for the Enantioselective Synthesis of a-Substituted b-Amino Acids”, Tetrahedron: Asymmetry, 14, 577-580 (2003).
- A.J. Mota, E. Castellano y E. Juaristi, “Simple Methodology for the Purification of Amino Acids”, Org. Prep. Proc. Int., 35, 414-417 (2003).
- E. Juaristi y R. Melgar-Fernández, y “a-Sodium Aldehydes, Ketones, and Other Na-C-CXY Compounds (Including Sodium Enolates)”, en “Science of Synthesis”, Houben-Weyl Methods of Molecular Transformations, George Thieme Verlag, Stuttgart (2005); capítulo 8.2.16, pp. 1285-1296.
- V.M. Mastranzo, E. Santacruz, G. Huelgas, E. Paz, M.V. Sosa-Rivadeneyra, S. Bernes, E. Juaristi, L. Quintero y C. Anaya de Parrodi, "Synthesis of Novel Chiral Ligands Containing the N-(S)-alpha-Phenylethyl Group and Their Evaluation as Activators in the Enantioselective Addition of Et2Zn to Benzaldehyde", Tetrahedron: Asymmetry, 17, 1663-1670 (2006).
- F. García-Flores, L. S. Flores-Michel y E. Juaristi, "Asymmetric Allylation of Benzoyl-hydrazones Promoted by Novel C2-Symmetric Bis-Sulfoxide Organocatalysts", Tetrahedron Lett., 47, 8235-8238 (2006).
- E. Juaristi, O. Muñoz-Muñiz y R. Melgar-Fernández,"alpha-Sodium Carboxylic Acids and Other Na-C-CXYZ Compounds (Including Sodium Enolates)", en "Science of Synthesis", Houben-Weyl Methods of Molecular Transformations, George Thieme Verlag, Stuttgart, (2006); chapter 8.2.15, pp. 1259-1284.
- M. Hernández-Rodríguezy E. Juaristi, “Structurally Simple ChiralThioureas as Chiral Solvating Agents in theEnantiodiscrimination of Carboxylic Acids”,Tetrahedron, 63, 7673-7678 (2007).
- R. Guzmán-Mejía, G. Reyes-Rangel y E. Juaristi, “Preparation of Chiral Derivatives of-Alanine Containing the -Phenylethyl Group:Useful Starting Materials for the AsymmetricSynthesis of -Amino Acids”, Nature Protocols,2, 2759-2766 (2007).
- G. Huelgas, S. Bernés, M. Sánchez, L.Quintero, E. Juaristi, C. Anaya de Parrodi yP.J. Walsh, “Synthesis and Dynamics ofAtropisomeric (S)-N-(-Phenylelthyl)benzamides”,Tetrahedron, 63, 12655-12664 (2007).
- R. Melgar-Fernández, R. González-Olvera, J.L.Olivares-Romero, V. González-López, L.Romero-Ponce, M. Ramírez-Zárate, P. Demare, I.Regla y E. Juaristi “Synthesis of NovelDerivatives of (1S,4S)-2,5-Diazabicyclo[2.2.1]heptaneand their Evaluation as Potential Ligands inAsymmetric Catalysis”, Eur. J. Org. Chem.,655-672 (2008).
- Y. Bandala, R. Melgar-Fernández, R.Guzmán-Mejía, J.L. Olivares-Romero, B.R. Díaz-Sánchez, R. González-Olvera y E. Juaristi,“Optimized Methodologies in Asymmetric Organic Synthesis Applying Microwaves”,J. Mex. Chem. Soc., 53,146-153 (2009).
- Y. Bandala, R. Melgar-Fernández, R. Guzmán-Mejía,J.L. Olivares-Romero, B.R. Díaz-Sánchez, R.González-Olvera y E. Juaristi, “MetodologíasOptimizadas en Síntesis Orgánica AplicandoMicroondas”, en “Aplicaciones de las Microondasen Química y en Biología”, El Colegio Nacional:México (2009); Cap?tulo 8, pp. 115-127.
- J.G. Hernández y E. Juaristi, “A GreenSysnthesis of ,- and,-Dipeptides underSolvent-Free Conditions”, J. Org. Chem.,75,7107-7111 (2010).
- H.A. Stefani, M.F.Z.J.Amaral, F. Manarin, R.A. Ando, N.C.S. Silva y E. Juaristi, “Functionalization of 2(S)-Isopropyl-5-iodo-pyrimidin-4-onesthrough Cu(I)-Mediated 1,3-Dipolar Azide-AlkyneCycloadditions”, Tetrahedron Lett., 52,6883-6886 (2011).
- J.G. Hernández, Víctor García López y E. Juaristi, “Solvent-Free Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-containing Thiodipeptides under Ball-Milling Conditions”, Tetrahedron, 68, 92-97 (2012).
- J. Vargas-Caporali, C. Cruz-Hernández, G. Dewynter y E. Juaristi,”Synthesis of Versatile Bifunctional Derivatives of Chiral Diamines Obtained Through Anchimerically Assisted Nucleophilic Substitution Reactions on diasteromeric Phenylprolinols”, Heterocycles, 86, 1275-1300 (2012).
- M. Escudero-Casao, A. Vega-Peñaloza y E. Juaristi, “Enantiopure 1,2,3-Triazo-Lyl--amino Acids via Click Cycloaddition Reaction on Racemic Alkynyl Precursors Followed by Separation of Stereoisomers”, Curr. Topics Med. Chem., 14, 1257-1270 (2014).
- A. Greco, R. de Marco, S. Tani, D. Giacomini, P. Galletti, A. Tolomelli, E. Juaristi y L. Gentilucci, “Convenient Synthesis of the Antibiotic Linezolid via a 1,3-Oxazolidin-2,4-dione Intermediate Derived from the Chiral Building Block Isoserine”, Eur. J. Org. Chem., pp 7614-7620, 2014.
- E. Juaristi, “Obras 4. Síntesis Orgánica y Química Heterocíclica”, El Colegio Nacional: México (2014), ISBN 978-607-729-073-0.
- S. Kumar, C. Cruz, S. Pal, R.K. Saunthwal, E. Juaristi y A.K. Verma, "An Expedient Tandem Approach to Benzothieno and Benzofuro-pyridines from o-Alkynyl Aldehydes via Silver-Catalyzed 6-endo-dig Ring Closure", J. Org. Chem., 80, 10548-10560 (2015).
- L.A. Polindara-García y E. Juaristi, "Synthesis of Ugi-4CR and Passerini-3CR Adducts Under Mechanochemical Activation", Eur. J. Org. Chem., 1095-1102 (2016).
- J.M. Landeros y E. Juaristi, "Mechanochemical Synthesis of Dipeptides Using Mg-Al Hydrotalcite as Catalyst Under Solvent-Free Reaction Conditions", Eur. J. Org. Chem., 687-694 (2017).
- M. Pérez-Venegas, G. Reyes-Rangel, A. Neri, J. Escalante y E. Juaristi, "Mechanochemical Enzymatic Resolution of N-Benzylated-ß3-Amino Esters", Beilstein J. Org. Chem., 13, 1728-1734 (2017).
- A. Espinoza-Vázquez, F.J. Rodríguez-Gómez, E. Juaristi, M. Escudero-Casao, D. Angeles-Beltrán, G.E. Negrón-Silva y M. Palomar-Pardavé, "Triazoles Derived from ß-Amino Acids as Corrosion Inhibitors for AP1 5L X52 Steel Immersed in 1M HC", Int. J. Electrochem. Sci., 13, 7517-7531 (2018).
- E. Juaristi y R. Notario, "Stereoelectronic Interactions Exhibited by 1JC-H One-Bond Coupling Constants and Examination of the Possible Existence of the Intramolecular a-Effect in Six-Membered Oxygen-Containing Heterocycles", J. Org. Chem., 83, 3293-3298 (2018).
- O. Sánchez-Antonio, R. González-Olvera, A. Aguilera-Cruz, A. Reyes-Ramírez y E. Juaristi, "Diastereoselective Synthesis of New Isoindolone-Derived Fused Heterocycles and Their Crystallographic Structures", Tetrahedron, 75, 130594 (2019).
- A. Fingerhut, J. Vargas-Caporali, M.A. Leyva-Ramírez, E. Juaristi y S.B. Tsogoeva, "Biominetic Non-Heme Iron(III) Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant", Molecules, 24, 3182 (2019).
- L. Alvarez-Santamaría, E. Juaristi, A.B. Arroyo-Colín, J. Palma-Flores y J. Escalante, "Efficient Solvent-Free Preparation of Imines, and Their Subsequent Oxidation with m-CPBA to Afford Oxaziridines", Green and Sustainable Chem., 9, 143-154 (2019).
- O. Sánchez-Antonio, R. González-Olvera, A. Aguilera-Cruz, A. Reyes-Ramírez y E. Juaristi, "Diastereoselective Synthesis of New Isoindolone-Derived Fused Heterocycles and Their Crystallographic Structures", Tetrahedron, 75, 130594 (2019).
- A. Fingerhut, J. Vargas-Caporali, M.A. Leyva-Ramírez, E. Juaristi y S.B. Tsogoeva, "Biominetic Non-Heme Iron(III) Catalyzed Epoxidation of Challenging Terminal Alkenes Using Aqueous H2O2 as an Environmentally Friendly Oxidant", Molecules, 24, 3182 (2019).
- L. Alvarez-Santamaría, E. Juaristi, A.B. Arroyo-Colín, J. Palma-Flores y J. Escalante, "Efficient Solvent-Free Preparation of Imines, and Their Subsequent Oxidation with m-CPBA to Afford Oxaziridines", Green and Sustainable Chem., 9, 143-154 (2019).
- M. Anselmi, P. Stavole, E. Boanini, A. Bigi, E. Juaristi y L. Gentilucci, "Solvent-Free and Minimal Solvent-Assisted Peptide Bond Formation Promoted by Nanocrystalline Hydroxyapatite", Future Medicinal Chem., 12, 479-491 (2020).
- M. Pérez-Venegas, M.N. Villanueva-Hernández, E. Peña-Cabrera y E. Juaristi, "Mechanochemically Activated Liebeskind-?Srogl (L-?S) Cross-?Coupling Reaction: Green Synthesis of meso-?Substituted BODIPYs", Organometallics, 39, 2561-2564 (2020).
- J.C. Jiménez-Cruz, R. Guzmán-Mejía, E. Juaristi, O. Sánchez-Antonio, M.A. García-Revilla, J.B. González-Campos y J. Aviña-Verduzco, "Preparation of aromatic ?-?hydroxyketones by means of Heck coupling of aryl halides and 2,?3-?dihydrofuran, catalyzed by a palladium(II) glycine complex under microwave irradiation", New Journal of Chemistry, 44, 13382-13392 (2020).
1.1 Lucía E. Valle A., “Análisis Conformacional del 1,3-Difenilfosfinoíl-1,3-ditiano y sus Derivados: Estudio de la Interacción Anomérica S-C-P”, CINVESTAV-IPN, marzo de 1987.
1.2 Roberto Martínez, “Análisis Conformacional y Electroquímica de 1,3-Dioxanos y 1,3-Ditianos Sustituídos en C(5)”, CINVESTAV-IPN, abril de 1987.
1.3 G. Bárbara Gordillo Román, “Análisis Conformacional de Ciclohexanos y de 1,3 Dioxanos Sustituidos por Grupos Sulfuro, Sulfinilo y Sulfonilo. Resultados Experimentales y Cálculos Teóricos”, CINVESTAV-IPN, noviembre de 1988.
1.4 Abelardo Flores Vela, “Análisis Conformacional y Espectroscópico del 2-Difenilfos- finoíl-1,3-dioxano, del 2-Difenilfosfinoíl-1,3-oxatiano y sus Derivados. Evaluación de la Interacción Anomérica O-C-P(O)”, CINVESTAV-IPN, noviembre de 1989.
1.5 Gabriel Cuevas González-Bravo, “Estudio de los Efectos Estereoelectrónicos Responsables del Comportamiento Conformacional de 1,3-Ditianos 2-Sustituidos, y Heterociclos Análogos”, CINVESTAV-IPN, marzo de 1993.
1.6 Delia Quintana Zavala, “Síntesis Asimétrica de ß-Amino Acidos a-Sustituídos a partir de la 1-Benzoíl-2(S)-tert-butil-3-metilperhidropirimidín-4-ona”, CINVESTAV-IPN, diciembre de 1994.
1.7 Jaime Escalante García, “Síntesis Enantioselectiva de ß-Amino Acidos a,ß- Disustituídos”, CINVESTAV-IPN, julio de 1995.
1.8 Domingo Madrigal Peralta, “Alquilación Diastereoselectiva de N-(a-Metilbencil)-1,3-imidazolidín-4-onas y sus Análogos”, CINVESTAV-IPN, febrero de 1996.
1.9 Cecilia Anaya Berrios, “Síntesis y Aplicación de Ligandos Quirales Incorporando el Grupo (S)-a-Metilbencilo, CINVESTAV-IPN, febrero de 1997.
1.10 Francisco Díaz Cedillo, “Análisis Conformacional de 1,3-Dioxanos 5-Sustituidos en Presencia de Sales”, CINVESTAV-IPN, agosto 15, 1997.
1.11 Oscar García Barradas, “Síntesis y Aplicación de Imidazolidinonas Quirales Conteniendo el Grupo Metilbencilo. Síntesis Enantioselectiva de Acidos Amino- Fosfónicos Quirales” CINVESTAV-IPN, Agosto 25, 1997.
1.12 Adelfo Natalio Reyes Ramírez, “Uso de la (S)-a-Metilbencilamina y Derivados Estructuralmente Relacionados en Procesos para la Obtención de Compuestos Enantiopuros”, CINVESTAV-IPN, Marzo 26, 1999.
1.13 Sandra Antúnez Perezache, “Aplicación de la Química Computacional en la Determinación de las Contribuciones Entálpicas y Entrópicas en Equilibrios Conformacionales de Ciclohexanos Sustituidos y Análogos Heterocíclicos”, CINVESTAV-IPN, Julio 2, 1999.
1.14 Yara Ramírez Quirós, “Estudio Conformacional de 1-Benzoílperhidropirimidin-4-onas a Partir de Datos de Difracción de Rayos-X”, CINVESTAV-IPN, Noviembre 5, 1999.
1.15 Heraclio López Ruiz, “Preparación de Imino Eteres Quirales y su Aprovechamiento en la Síntesis Enantioselectiva de Derivados del Acido Aspártico”, CINVESTAV-IPN, Septiembre 14, 2000.
1.16 José Luis León Romo, “Síntesis Enantioselectiva de a-Amino Acidos Vía Benzodiazepinonas Quirales”, CINVESTAV-IPN, Octubre 31, 2000.
1.17 Víctor Manuel Gutiérrez García, “Síntesis Enantioselectiva de ß-Aminoácidos y Acidos Carboxílicos a-Sustituidos, Usando como Auxiliares Quirales la (S)- y (R)-a-Feniletilamina”, CINVESTAV-IPN, Septiembre 6, 2002.
1.18 Jesús Samuel Cruz Sánchez, “Síntesis y Análisis Conformacional de 1,3-Ditianos Sustituidos en C(5) con Grupos Polares”, CINVESTAV-IPN, Diciembre 13, 2002.
1.19 Martha V. Sosa Rivadeneyra, “Síntesis de ß-Aminoalcoholes Derivados de la (S)-1-Fenil-etilamina y su Aplicación en Síntesis Asimétrica”, Universidad Autónoma de Puebla (Co-dirección con la Dra. Leticia Quintero Cortés), Marzo 14, 2003.
1.20 Irma Linzaga Elizalde, “Síntesis Estereoselectiva de Precursores Quirales de Acidos a-Aminofosfónicos,” CINVESTAV-IPN, Marzo 19, 2003.
1.21 Judit Araceli Aviña Verduzco, “Síntesis en Solución y en Fase Sólida de a,ß- y ß-Péptidos No Naturales. Análisis Conformacional, Reacciones de Alquilación y Afinidad a Iones Metálicos”, CINVESTAV-IPN, Junio 8, 2004.
1.22 Virgina F. Marañón Ruiz, “Síntesis y Caracterización de Derivados de (1S,2S,1’S)- y (1R,2R,1’S)-2-N-[(a-Metilbencil)amino]ciclohexanol como Precursores de Ligantes y Auxiliares Quirales”, CINVESTAV-IPN, Junio 11, 2004.
1.23 Gloria Elizabeth Moreno Morales, “Preparación de Derivados de Fósforo a Partir de N,N’-Di-(a-Feniletil)-1,2-diaminas y sus Aplicaciones”, Universidad Autónoma de Puebla, (Co- dirección con la Dra. Cecilia Anaya Berrios), Julio 16, 2004.
1.24 Elena Castellanos Santiago, “Síntesis Enantioselectiva de a,ß-Diaminoácidos”, CINVESTAV-IPN, Septiembre 10, 2004.
1.25 Maribel Vázquez Hernández, “Síntesis y Análisis Conformacional de 1,3-Dioxanos 2,5-Disustituidos en Presencia de Sales Metálicas”, Cinvestav-IPN, Julio 6, 2005.
1.26 Claudia Gabriela Avila Ortiz, “Síntesis Enantioselectiva de ß-Amino-ácidos. Obtención de los Análogos ß2 de la Dopa, Selenocisteína y Cisterna”, Cinvestav-IPN, Septiembre 7, 2005.
1.27 Marcos Hernández Rodríguez, “Síntesis de Ureas y Tioureas Quirales, y su Aplicación como Organocatalizadores, Ligantes y Agentes Solvatantes Quirales”, Cinvestav-IPN, Septiembre 6, 2005.
1.28 Roberto C. Melgar Fernández, “Síntesis y Aplicación en Catálisis Asimétrica de Derivados de (1S,4S)-2,5-Diazabiciclo[2.2.1]heptanos, Pirazolidinonas Quirales y Compuestos que Incorporan el Fragmento a-Feniletilo”, Cinvestav-IPN, Junio 12, 2007.
1.29 Blanca Rosa Díaz Sánchez, “Aplicaciones de Compuestos Organometálicos Derivados de la Asparagina en Síntesis Asimétrica de ß-Aminoácidos a Sustituidos”, Cinvestav-IPN, Enero 30, 2008.
1.30 Fred García Flores, “Síntesis de bis-Sulfóxidos Quirales de Simetría C2 y su Aplicación en Síntesis Asimétrica”, Cinvestav-IPN, Agosto 21, 2008.
1.31 José Luis Olivares Romero, “Síntesis de Diaminas Quirales Derivadas de (S)-Prolina y su Aplicación en Síntesis Asimétrica”, Cinvestav-IPN, Enero 27, 2009.
1.32 Yamir Bandala Solano, “Síntesis en Soluciòn y en Fase Sólida de a/ß-Tetrapéptidos y Ciclo-ß-Dipéptidos. Análisis Conformacional y Modelado Molecular”, Cinvestav-IPN, Marzo 30, 2009.
1.33 Rodrigo González Olvera, “Síntesis de Derivados del (1S,4S)-2,5-Diazabiciclo [2.2.1]heptano y su Aplicación en Organocatálisis Asimétrica”, Cinvestav-IPN, Agosto 11, 2011.
1.34 José Gregorio Hernández Barajas, “Mecanoquímica como Alternativa Verde en la Síntesis Orgánica y en la Organocatálisis Asimétrica”, CINVESTAV-IPN, Agosto 17, 2012.
1.35 Erika Jiménez González, “Síntesis de Ciclodipéptidos a Partir de a- y ß-Aminoácidos, Análisis Conformacional y Alquilación Diastereoselectiva”, CINVESTAV-IPN, Diciembre 7, 2012.
1.36 Alberto Vega Peñaloza, “Diseño y Síntesis de Organocatalizadores Quirales Derivados de Aminoácidos y su Aplicación en las Reacciones Aldólica y Michael Asimétricas”, CINVESTAV-IPN, Enero 31, 2014.
1.37 Margarita del Rosario Escudero Casao, “Síntesis de ß-Aminoácidos Enantioméricamente Puros: ß2-Homo-tert-Leucina, ß2-Aminoácidos 1,2,3-Triazolil Sustituidos y ß2-homo-Histidina, así como su Aplicación como Bloques de Construcción de a/ß-Dipéptidos”, CINVESTAV-IPN, Enero 23, 2015.
1.38 Jorge Vargas Caporali, "Síntesis, Caracterización y Funcionalización de Diaminas Vecinales Quirales Derivadas de (S)-Prolina y su Aplicación en Catálisis Asimétrica", CINVESTAV-IPN, Septiembre 8, 2015.
1.39 Elizabeth Machuca de la Paz, "Síntesis y Evaluación de Organocatalizadores a,a- y a,ß-Dipeptídicos Conteniendo (S)-Prolina en la Reacción Aldólica Asimétrica. Estrategias para la Aplicación de Química Verde", CINVESTAV-IPN, Junio 2, 2016.
1.40 Luis Arturo Obregón Zúñiga, “Diseño y Síntesis de Líquidos Iónicos Quirales Derivados de Aminoácidos y su Aplicación como Organocatalizadores en Reacciones Orgánicas Asimétricas”, CINVESTAV-IPN, Diciembre 14, 2017.
1.41 Carlos Alberto Cruz Hernández, "Diseño, Síntesis y Evaluación de Organocatalizadores a Base de Fosforamidas Quirales Incorporando el Fragmento de (R)- o (S)-Prolina para Procesos en Medio Acuoso", CINVESTAV-IPN, septiembre 20, 2018.
1.42 Mario Pérez Venegas, “Activación mecánica como alternativa verde en biocatálisis y síntesis asimétrica”, CINVESTAV-IPN, agosto 21, 2020.