Publicaciones Recientes
B. Rodriguez-Molina, A. Pozos, R. Cruz, M. Romero, B. Flores, N. Farfán, R.Santillan, M. A.Garcia-Garibay. Synthesis and Solid State Characterization of Steroid-based Molecular Rotors, Org. Biomol. Chem. 8, 2993–3000 (2010).
J.O.H. Pérez-Díaz, J. L. Vega-Baez, J. Sandoval-Ramírez, S. Meza-Reyes, S. Montiel-Smith, N. Farfán, R. L. Santillan. Novel steroidal penta-and hexacyclic compounds derived from 12-oxospirosan sapogenins Steroids 75, 1127–1136(2010).
B. Rodríguez-Molina, N. Farfán, M. Romero, J. M. Méndez-Stivalet, R. Santillan,M.A. Garcia-Garibay. Anisochronous Dynamics in Crystalline Arrays of Steroidal Molecular Rotors: A Dynamic Phase Transition within Helical Stacks, J. Amer.Chem. Soc. 133(19), 7280–7283 (2011.)
O. Domínguez, B. Rodríguez-Molina, M. Rodríguez, A. Ariza, N. Farfán, R. Santillan. X-ray crystallographic and spectroscopic properties of eight Schiff bases as evidence of the proton transfer reaction. Role of the intermolecular hydrogen bond. New J. Chem. 35, 156-164 (2011).
D. Czajkowska-Szczykowska, B. Rodríguez-Molina, N.E. Magaña-Vergara, R. Santillan, J.W. Morzycki, M. A. Garcia-Garibay. Macrocyclic Molecular Rotors with Bridged Steroidal Frameworks. J. Org. Chem. 77, 9970-9978 (2012).
I:Jastrzebska, M. Górecki, J. Frelek, R. Santillan, L. Siergiejczyk, J.W. Morzycki “Photoinduced isomerization of 23-oxosapogenins:conformational analysis and spectroscopic characterization of 22-isosapogenins” J. Org. Chem. 77, 11257-11269 (2012).
Y. López, L. Rodríguez, R.E. del Río, N. Farfán, J.W. Morzycki, R.Santillan. “Regioselective cleavage of 22-oxo-23-spiroketals. Novel cholestanic frameworks with pyranone and cyclopentenone E rings on the side chain”. Steroids 77, 534-541 (2012).
J.O.H. Pérez-Díaz, L. Rárová, J.P. Muñoz Ocampo, N.E. Magaña-Vergara, N. Farfán, M. Strnad, R. Santillán. "Synthesis and Biological activity of 23- Ethylidene-26-hydroxy-22-oxocholestane derivatives from spirostanic sapogenins”. Europ. J. Med. Chem 51, 67-78 (2012).
P. Ramírez-Montes, Ma. E. Ochoa, V. Rodríguez, R, Santillan, H. García-Ortega, P. Rodríguez, N. Farfán. “Synthesis, characterization and X-ray analysis of new N,N’-disubstituted-1,4-diazepanes”. Tet. Lett. 53, 5887-5890 (2012).
N.E. Magaña-Vergara, L. Rarova, D. Soto-Castro, N. Farfan, M. Strnad, R. Santillan. “Synthesis and antiproliferative activity of novel steroidal dendrimer conjugates”. Steroids 78, 1254–1262 (2013).
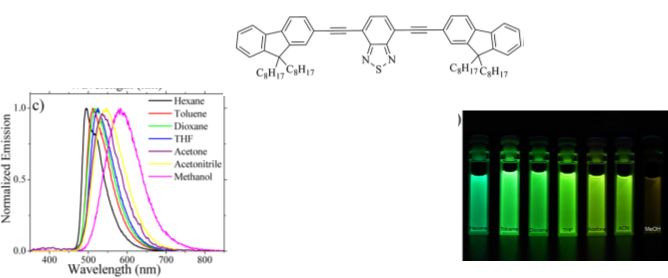
J. Rodríguez-Romero, L. Aparicio-Ixta, M. Rodríguez, G. Ramos-Ortíz, J.L. Maldonado, A. Jiménez-Sánchez, N. Farfán, R. Santillan. “Synthesis, chemical-optical characterization and solvent interaction effect of novel fluorene-chromophores with D-A-D structure”. Dyes and Pigments 98, 31-41 (2013).
D. Soto-Castro, N. E. Magaña-Vergara, N. Farfán, R. Santillan. Synthesis of steroidal dendrimers modified by ‘click’ chemistry with PAMAM dendrons as unimolecular micelles.Tetrahedron Letters 55, 1014–1019. (2014)
D. Czajkowska-Szczykowska, I. Jastrzebska, R. Santillan, J. W. Morzycki. The Synthesis of Disteroidal Macrocyclic Molecular Rotors by an RCM Approach. Tetrahedron 70, 9427-9435 (2014).
P. I. Ramirez-Montes, M. E. Ochoa, R. Santillan, D. J. Ramírez, N. Farfán. Steroidal Wheel-and-Axle Host Type Molecules: Insights from Awkward Shape, Conformation, Z′ > 1 and Packing. Cryst. Growth Des. 14, 4681−4690 (2014).
A. Corona Díaz, J.P. García Merinos, Y. López, J. B. González Campos, R.E. del Río, R. Santillan, N. Farfán, J. W. Morzycki. Regio- and stereoselective cleavage of steroidal 22-oxo-23-spiroketals catalyzed by BF3.OEt2. Steroids 100, 36-43 (2015).
S. Pérez-Estrada, B. Rodríguez-Molina, L. Xiao, R. Santillan, G. Jiménez-Osés, K.N. Houk, M.A. García-Garibay. Thermodynamic evaluation of Aromatic CH/? interactions and rotational entropy in a molecular rotor. J. Amer. Chem. Soc., 137, 2175-2178 (2015).
A.Jiménez-Sánchez, B. Ortíz, V. Ortíz Navarrete, J.C. Flores, N. Farfán, R. Santillán. A dual-model fluorescent Zn2+/Cu2+ ions sensor with in-situ detection of S2-/(PO4)- and colorimetric detection of Fe2+ ion. Inorg Chim. Acta 429, 243-251 (2015).
R. Arcos-Ramos, B.Rodriguez-Molina, E.González-Rodriguez, P.I.Ramirez Montes, Ma. E.Ochoa, R.Santillan, N.Farfán, M.A. Garcia-Garibay Crystalline arrays of molecular rotors with TIPStrityl and phenolic-trityl stators using phenylene, 1,2-difluorophenylene and pyridine rotators. RSC Adv. 5, 55201-55208. (2015).
A.Jiménez-Sánchez, B.Ortíz, V.Ortiz Navarrete, N.Farfán, R.Santillan “Two fluorescent Schiff base sensors for Zn2+: the Zn2+/Cu2+ ion interference”. Analyst 140, 6031-6039 (2015).
A.Jime´nez-Sa´nchez, N.Farfa´n, R.Santillan “Multiresponsive Photo-, Solvato Acido-, and Ionochromic Schiff Base Probe”. J. Phys. Chem. C 119,13814−13826 (2015).
C. Jiménez, N. Farfan, M. Romero-Avila, R. Santillan, I. Malfant, P. G. Lacroix, Light induced nonlinear optical switch in boronated chromophores: A theoretical search towards high contrast switches in the azobenzene series. J. Organomet. Chem., 799-800, 215-222 (2015).
P. Labra-Vázquez, M. Palma-Contreras, R. Santillan, N. Farfán. Vibrational, structural and electronic study of a pyridinium salt assisted by SXRD studies and DFT calculations. J. Molec. Struct., 1131, 156-162 (2017).https://doi.org/10.1016/j.molstruc.2016.11.033
N. Aguilar-Valdez, M. Maldonado-Domínguez, R. Arcos-Ramos, M. Romero- Ávila, R. Santillan, N. Farfán. Synthesis of steroidal molecular compasses: exploration of the controlled assembly of solid organic materials. CrystEngComm.,19,1771-1777(2017).
https://doi.org/10.1039/c7ce00157f
D. Soto-Castro, R. Carlos Lara Contreras, Ma. del S. Pina Canseco, R. Santillán, Ma. T. Hernández-Huerta, G. E. Negrón Silva, E. Pérez-Campos, S. Rincón. Solvent-free synthesis of 6 beta-phenylamino-cholestan-3-beta, 5-alpha-diol and (25R)-6-beta-phenylaminospirostan-3-beta,5-alpha-diol as potential antiproliferative agents. Steroids126, 92-100(2017).
https://doi.org/10.1016/j.steroids.2017.08.008
T. Guerrero, R. Santillan, H. García-Ortega, O. G. Morales Saavedra, N. Farfán and P. G. Lacroix. Bis(4-nitroanilines) in interactions through a p-conjugated bridge: conformational effects and potential molecular switches. New J. Chem., 41,11881-11890 (2017). https://doi.org/10.1039/c7nj02622f
M. Ibarra-Rodríguez, B. M. Mun~oz-Flores, H. V. Rasika Dias, M. Sanchéz, A. Gomez-Trevin~o, R. Santillan, N. Farfán, and V. M. Jiménez-Pérez. Fluorescent Molecular Rotors of Organoboron Compounds from Schiff Bases: Synthesis, Viscosity, Reversible Thermochromism, Cytotoxicity, and Bioimaging Cells. J. Org. Chem., 82, 2375−2385 (2017).
https://doi.org/10.1021/acs.joc.6b02802
N. E. Magaña-Vergara, D. Soto-Castro, H. Vazquez-Lima, R. Yépez, and R. Santillan. Synthesis of Fréchet-type poly(aryl ether) dendrimers withallyl end groups: comparative convergent and divergent approaches.Arkivoc part v, 117-128 (2017).
https://doi.org/10.24820/ark.5550190.p009.994
A. Jiménez-Urias, A. Zaavik Lugo-Aranda, M. Miranda-Olvera, N. Farfán, R. Santillan, R. Arcos-Ramos, Ma. del P. Carreón-Castro. Synthesis and characterization of dumbbell-like BTD-based derivatives to engineer organic building blocks in solid-state. J. Molec. Struct., 1153, 34-41(2018).
https://doi.org/10.1016/j.molstruc.2017.09.106
A. Enríquez-Cabrera, A. Vega-Peñaloza, V. Álvarez Venicio, M. Romero-Ávila, P. G. Lacroix, G. Ramos-Ortiz, R. Santillan, N. Farfán. Two-photon absorption properties of four new pentacoordinated diorganotin complexes derived from Schiff bases with fluorene. J. Organomet. Chem., 855, 51-58 (2018). https://doi.org/10.1016/j.jorganchem.2017.12.014
H. Briseño-Ortega, L. Juárez-Guerra, S. Rojas-Lima, L. H. Mendoza-Huizar, R. A. Vázquez-García, N. Farfán, R. Arcos-Ramos, R Santillan, H. López-Ruiz. One pot synthesis, X-ray crystal structure of 2-(20-hydroxyphenyl) oxazolo[4,5-b]pyridine derivatives and studies of their optical properties. J. Molec. Struct., 1157, 119-126 (2018).
https://doi.org/10.1016/j.molstruc.2017.12.059
T. Runka, K. Olszewska, P. Fertsch, A. Lapinski, I. Jastrzebska, R. Santillan, N. Farfán. Vibrational spectroscopic characterization of cyclic and acyclic molecular rotors with 1,4-diethynylphenylene-d4 rotators. Spectrochim. Acta Part A: Molecular and Biomolecular Spectroscopy, 192, 393–400 (2018).
https://doi.org/10.1016/j.saa.2017.11.052
M. Cantón-Díaz, B. M. Muñoz-Flores, I. Moggio, E. Arias, A. de León, Ma. C. Garcia-López, R. Santillán, Ma. E. Ochoa, V. M. Jiménez-Pérez. One-pot microwave-assisted synthesis of organotin Schiff bases: an optical and electrochemical study towards their effects in organic solar cells.New J. Chem., 42, 14586-14596 (2018).
https://doi.org/10.1039/c8nj02998a
Ma. E. Ochoa, P. Labra-Va´zquez, N. Farfa´n, R. Santillan. Designed Synthesis and Crystallization of Isomorphic Molecular Gyroscopes with Cell-like Bilayer Self-Assemblies. Cryst. Growth Des., 18, 2795−2803 (2018).https://doi.org/10.1021/acs.cgd.7b01542
C. I. Bautista-Hernandez, G. E. Negron-Silva, R. Santillan, B. I. Vergara-Arenas, D. Angeles-Beltran, L. Lomas-Romero, D. Perez-Martinez.Design and synthesis of new carbohydrate-lithocholic acid conjugates linked via1,2,3-triazole rings. Tetrahedron 74, 2009-2019 (2018).https://doi.org/10.1016/j.tet.2018.03.0087
J. Ordóñez-Hernández , A. Jiménez-Sánchez, H. García-Ortega , N.Sánchez-Puig, M. Flores-Álamo, R. Santillan, N. Farfán. A series of dual-responsive Coumarin-Bodipy probes for local microviscosity monitoring. Dyes and Pigments 157, 305–313 (2018).
https://doi.org/10.1016/j.dyepig.2018.05.009
M. Ibarra-Rodríguez, B. M. Muñoz-Flores, J. Lara Cerón, R. Santillan, Ma. E. Ochoa, N. Waksman, V. M. Jiménez-Pérez. Centrosymmetric Binuclear Boron Compounds Derived from Dithiooxamides: Synthesis, Characterization, and Their Photophysical Properties. Journal of Chemistry Volume 2018, On line Article ID 4295970, 10 pages
https://doi.org/10.1155/2018/4295970
C. C. Jiménez, A. Enríquez-Cabrera, O. González-Antonio, J. Ordóñez-Hernández, P. G. Lacroix, P. Labra-Vázquez, N. Farfán, R. Santillan. State of the Art of Boron and Tin Complexes in Second- and Third-Order Nonlinear Optics. Inorganics 6, 131 (2018) (Review) https://doi:10.3390/inorganics6040131
A. Corona-Díaz, J. P. García-Merinos, M. E. Ochoa , R. E. del Río , R. Santillan , S. Rojas-Lima , J. W. Morzycki , Y. López, TiCl4 catalyzed cleavage of (25R)-22-oxo-23-spiroketals. Synthesis of sapogenins with furostanol and pyranone E rings on the side chain. Steroids 152108488 (2019).
https://doi.org/10.1016/j.steroids.2019.108488
A. Molina-Paredes, V. M. Jiménez-Pérez, J. A. Lara-Cerón, I. Moggio E. Arias, R. Santillán, M. Sánchez, A. Saucedo-Yañez, B. M. Muñoz-Flores. Fluorescent boron Schiff bases dyes for staining silk fibroin: Green synthesis, structural characterization, DFT, and photophysical properties. Applied Organomet. Chem., 33: e4609 (2019) https://doi.org/10.1002/aoc.4609
I. Rojas-León, H. Alnasr, K. Jurkscha, M. G. Vasquez-Ríos, G. Gomez-Jaimes, H. Ho¨pfl, I. F. Hernández-Ahuactz and R. Santillan. Formation of Metal-Based 21- and 22-Membered Macrocycles from Dinuclear Organotin Tectons and Ditopic Organic Ligands Carrying Carboxylate or Dithiocarbamate Groups. Organometallics 38, 2443−2460 (2019). https://doi.org/10.1021/acs.organomet.9b00132
J. Ordóñez-Hernández, R. Arcos-Ramos, H. García-Ortega, E. Munguía-Viveros, M. Romero-Ávila, M. Flores-Alamo, I. Gracia-Mora, F. Sánchez-Bartéz, R. Santillan, N. Farfán. Synthesis and structural analysis of bioactive Schiff-base pentacoordinated diorganotin(IV) complexes. J. Molec. Struct., 1180 462-471(2019).
https://doi.org/10.1016/j.molstruc.2018.11.107
J. Ordóñez-Hernández, R. Arcos-Ramos, H. García-Ortega, E. Munguía-Viveros, M. Romero-Ávila, M. Flores-Alamo, I. Gracia-Mora, F. Sánchez- Bartéz, R. Santillan, N. Farfán. Synthesis and structural analysis of bioactive Schiff-base pentacoordinated diorganotin(IV) complexes. J. Molec. Struct., 1180 462-471(2019).
https://doi.org/10.1016/j.molstruc.2018.11.107
Ma. E. Ochoa, R. Arcos-Ramos, P. I. Ramirez-Montes, H. Ho¨pfl, M. A. Leyva, N. Farfán, R. Santillan. Asymmetric Molecular Rotors Based on Steroidal Fragments. Organic Building Blocks Displaying Versatile Supramolecular Steroid-Stacking. Cryst. Growth Des. 19, 6114−6126 (2019).
https://doi.org/10.1021/acs.cgd.9b00238
O. González-Antonio, M. Navarro Villalobos, Ma. M. Vázquez-Alvarado, R. Santillan, B. Flores-Pérez, M. Romero-Ávila, N. Farfán. On the nucleophilic derivatization of 4,7-dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine: basis for biologically interesting species and building blocks for organic materials. New J. Chem., 43, 10491-10500 (2019).
https://doi.org/10.1039/c9nj01855g
M. Ibarra-Rodríguez, B. M. Muñoz-Flores, J. Lara-Cerón, R. Santillán, María E. Ochoa, M. Sánchez, V. M. Jiménez-Pérez. Synthesis, Characterization, X-Ray Structure, and Conformation DFT Calculation of a Carbohydrazide Derivative J. Chem. Cryst. 49:92–97 (2019).
https://doi.org/10.1007/s10870-018-0740-4
J. C. Berrones-Reyes, B. M. Muñoz-Flores, A. C. Uscanga-Palomeque, R. Santillán, C. Del Angel-Mosqueda, D. Nobis, M. A. Cochrane, S. W. Magennis, and V. M. Jiménez-Pérez.Two-Photon Detection of Organotin Schiff Base Complexes in Cancer Cells. ChemistrySelect 5, 1623–1627 (2020).
https://doi.org/10.1002/slct.201904816
N. Aguilar-Valdez, N. Esturau-Escofet, O. González-Antonio, M. Romero-Ávila, B. Flores-Pérez, M. A. Leyva, D. Díaz, R. Santillan, N. Farfán. Synthesis, complete NMR assignment and structural study of a steroidal dimer of 17a-ethynyl-5a,10a-estran-17ß-ol with diethynylbenzene spacer. Steroids 157, 108606 (2020).
https://doi.org/10.1016/j.steroids.2020.108606
T. Pawlak, D. Czajkowska-Szczykowska, I. Jastrzebska, R. Santillan, B. Seroka, J. Maj, Jadwiga, J. Morzycki, P. Labra-Vázquez, N. Farfán, G. Bujacz, M. J. Potrzebowski. Influence of Hydrogen/Fluorine Substitution on Structure, Thermal Phase Transitions and Internal Molecular Motion of AromaticResidues in the Crystal Lattice of Steroidal. Cryst. Growth & Design 20, 2202−2216 (2020).
https://dx.doi.org/10.1021/acs.cgd.9b01179
P. Labra-Vázquez, R. Flores-Cruz, A. Galindo-Hernández, J. Cabrera, C. Guzmán-Cedillo, A. Jiménez-Sánchez, P. G. Lacroix, R. Santillan, N. Farfán, R. Núñez, Tuning the cell uptake and subcellular distribution in BODIPY carboranyl dyads: An experimental and theoretical study Chemistry– Chem. A Europ. J. 26, 16530 – 16540 (2020).
https://dx.doi.org/10.1002/chem.202002600
M. Romero Ávila, A. F. León Rojas, P. Lacroix, I. Malfant, N. Farfán, R. Mhanna, R. Santillan, G. Ramos-Ortiz, J.-P. Malval. Two-Photon Triggered NO-Release via a Ruthenium-Nitrosyl Complex with a Star-Shaped Architecture J. Phys. Chem. Lett. 11(16), 6487-6491(2020).
https://dx.doi.org/10.1021/acs.jpclett.0c01953
M. Farfán-Paredes, O. González-Antonio, D. E. Tahuilán-Anguiano, J. Peón, A. Ariza; P. G. Lacroix, R. Santillan, N. Farfán. Physicochemical and computational insight of 19F NMR and emission properties of meso-(o-aryl)-BODIPYs. New J. Chem., 44, 19459 - 19471 (2020). https://doi.org/10.1039/D0NJ02576C
T. Roman, D. Ramirez, R. Fierro-Medina, R. Santillan, N. Farfán. Ferrocene and Organotin (IV) Conjugates Containing Amino Acids and Peptides: A Promising Strategy for Searching New Therapeutic and Diagnostic Tools. Curr. Org. Chem. 24, 1-22 (2020). https://doi.org/10.2174/1385272824999201001154259
K. Olszewska, I. Jastrzebska, A. Lapinski, M. Górecki, R. Santillan, N. Farfán, T.Runka. Steroidal Molecular Rotors with 1,4-diethynylphenylene Rotators: Experimental and Theoretical Investigations Towards Seeking Efficient Properties. J. Phys. Chem. B 124, 9625−9635 (2020).
https://dx.doi.org/10.1021/acs.jpcb.0c06464
O. González-Antonio, R. Yépez, M. M. Vázquez-Alvarado, B. Flores-Pérez, N. Farfán, C. Amador-Bedolla, M. Romero-Ávila, R. Santillan. Assessing electronic properties of desymmetrized heterocyclic patterns: towards tuning small molecules for photovoltaic applications. MRS Advances 5, 3171–3184 (2020).
http://dx.doi.org/10.1557/adv.2020.434.
J. C. Berrones-Reyes, B M. Muñoz-Flores, A. C. Uscanga-Palomeque, R. Santillán, C. Del Angel-Mosqueda, D. Nobis, M. A. Cochrane, S. W. Magennis and V. M. Jiménez-Pérez. Two-Photon Detection of Organotin Schiff Base Complexes in Cancer Cells. ChemistrySelect 5, 1623-16-27 (2020).
https://doi.org/10.1002/slct.201904816
L. M Carrillo-Cocom, B. B Villagómez González, R. Santillan, D. Soto-Castro, P. M. Sánchez Ocampo, A. Zepeda and J. Capataz Tafur. Synthesis of diosgenin prodrugs: anti-inflammatory and antiproliferative activity evaluation J. Chem. Sci. 132:104 (2020). https://doi.org/10.1007/s12039-020-01808-y
A. Arenaza-Corona, M. D. Couce-Fortúnez, A. de Blas, D. Morales-Morales, R. Santillan, H. Ho¨pfl, T. Rodríguez-Blas and V. Barba. Further Approaches in the Design of Antitumor Agents with Response to Cell Resistance: Looking toward Aza Crown Ether-dtc Complexes : Inorg. Chem. 59, 15120−15134 (2020).
https://dx.doi.org/10.1021/acs.inorgchem.0c02068
P. Sánchez-Portillo, A. Hernandez-Sirio, C. Godoy-Alcantar, P. G. Lacroix, V. Agarwal, R. Santillan, V. Barba. Colorimetric metal ion (II) Sensors Based on imine boronic esters functionalized with pyridine. Dyes and Pigments 186, 108991 (2021).
https://doi.org/10.1016/j.dyepig.2020.108991
V. Bukhanko, A. Felipe León-Rojas, P. G. Lacroix, M. Tassé, G. Ramos-Ortiz, R. M. Barba-Barba, N. Farfán, R. Santillan, and I. Malfant. Two-Photon Absorption Properties in “Push-Pull” Ruthenium Nitrosyl Complexes with various Fluorenylterpyridine-Based Ligands. Eur. J. Inorg. Chem. 1670–1684 (2021).
https://doi.org/doi.org/10.1002/ejic.202100109
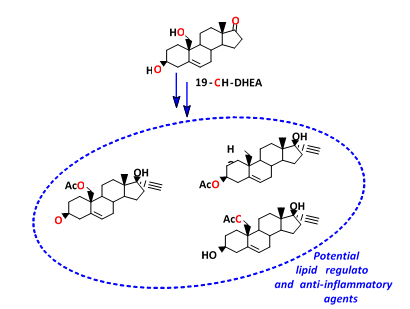
Ma. Eugenia Ochoa, Norberto Farfán, Pablo Labra-Vázquez, Delia Soto-Castro, Rosa Santillan. Synthesis, characterization and in silico screening of potential biological activity of 17a-ethynyl-3ß, 17ß, 19-trihydroxyandrost-5-en acetylated derivatives. J. Molec. Struct. 1225, 129167 (2021).
https://dx.doi.org/10.1021/acs.inorgchem.0c02068
L. Loaeza, R. Corona-Sanchez, G. Castro, M. Romero-Avila, R. Santillan, V. Maraval, R. Chauvin, N. Farfan. Synthesis and optical properties of 1-ethyl-indol-3-yl-substituted azaBODIPY dyes at the 1,7-positions.Tetrahedron 83, 131983 (2021).
https://doi.org/10.1016/j.tet.2021.131983
1.Synthesis, characterization and in silico screening of potential biological activity of 17a-ethynyl-3ß, 17ß, 19-trihydroxyandrost-5-en acetylated derivatives. Ma. Eugenia Ochoa, Norberto Farfán, Pablo Labra-Vázquez, Delia Soto-Castro, Rosa Santillan. J. Molec. Struct. 1225, 129167 (2021).
https://doi.org/10.1016/j.molstruc.2020.129167
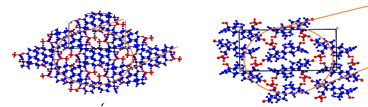
2. Assessing electronic properties of desymmetrized heterocyclic patterns: towards tuning small molecules for photovoltaic applications. O. González-Antonio, R. Yépez, M. M. Vázquez-Alvarado, B. Flores-Pérez, N. Farfán, C. Amador-Bedolla, M. Romero-Ávila, R. Santillan. MRS Advances 5, 3171–3184 (2020).
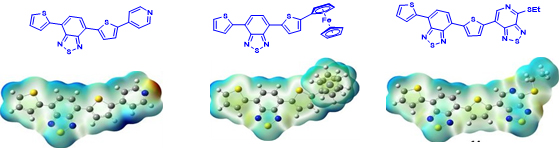
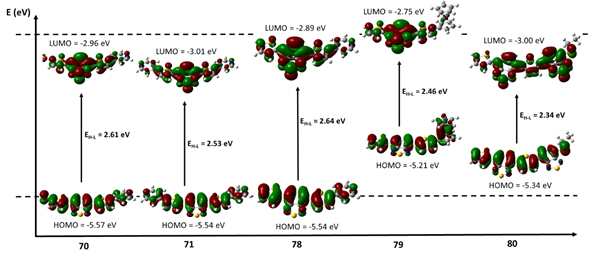
3. Influence of Hydrogen/Fluorine Substitution on Structure, Thermal Phase Transitions and Internal Molecular Motion of Aromatic Residues in the Crystal Lattice of Steroidal Rotors. T. Pawlak, D. Czajkowska-Szczykowska, I. Jastrzebska, R. Santillan, B. Seroka, J. Maj, Jadwiga, J. Morzycki, P. Labra-Vázquez, N. Farfán, G. Bujacz, M. J. Potrzebowski. Cryst. Growth & Design 20, 2202−2216 (2020).
https://dx.doi.org/10.1021/acs.cgd.9b01179
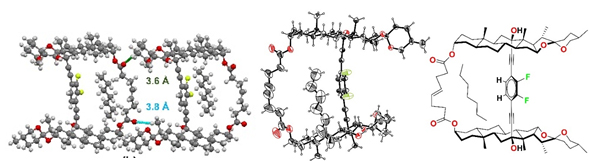
4. Designed Synthesis and Crystallization of Isomorphic Molecular Gyroscopes with Cell-like Bilayer Self-Assemblies Ma. E. Ochoa, P. Labra-Va´zquez, N. Farfa´n, R. Santillan. . Cryst. Growth Des., 18, 2795−2803 (2018)
DOI: 10.1021/acs.cgd.7b01542

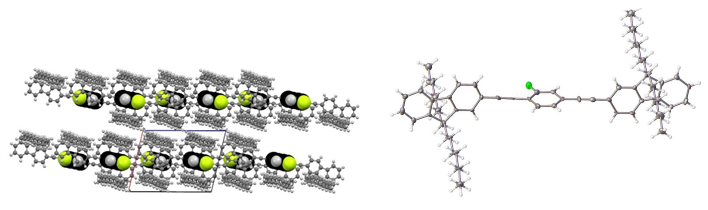
5. Synthesis, structure and local molecular dynamics for crystalline rotors based on hecogenin/botogenin steroidal frameworks I. Jastrzebska, T. Pawlak, R. Arcos-Ramos, E. Florez-Lopez, N. Farfán, D. Czajkowska-Szczykowska, J. Maj, R. Santillan, J. Morzycki, M. Potrzebowski. Cryst. Growth Des. 16, 5698-5709 (2016)
DOI:10.1021/acs.cgd.6b00726
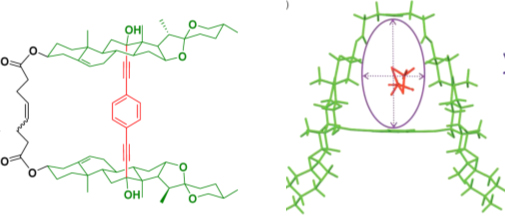
6. Synthesis, chemical optical characterization and solvent interaction effect of novel fluorene-chromophores with D-A-D structure J. Rodríguez-Romero, L. Aparicio-Ixta, M. Rodríguez, G. Ramos-Ortíz, J.L. Maldonado, A. Jiménez-Sánchez, N. Farfán, R. Santillan. Dyes and Pigments 98, 31-41 (2013).
DOI:10.1016/j.dyepig.2012.12.029
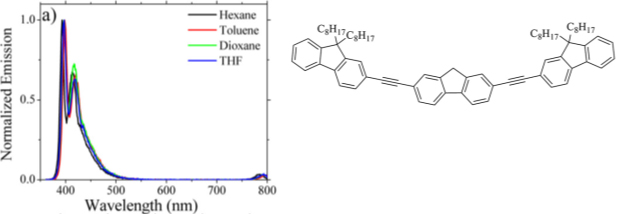
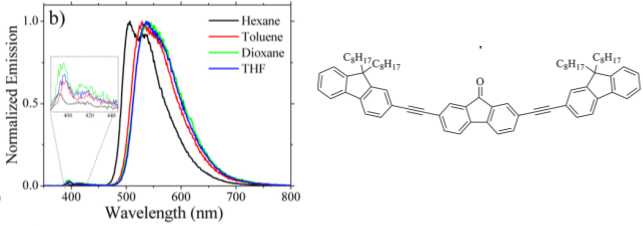
7. Photoinduced isomerization of 23-oxosapogenins: conformational analysis and spectroscopic characterization of 22-isosapogenins I. Jastrzebska, M. Górecki, J. Frelek, R. Santillan, L. Siergiejczyk, J.W. Morzycki. J. Org. Chem. 77, 11257-11269 (2012).
doi.org/10.1021/jo3022549